Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6676
Revised: April 6, 2005
Accepted: April 9, 2005
Published online: November 14, 2005
AIM: To examine the sensory and motor response(s) of the stomach following fundic distention and to assess whether cholinergic mechanisms influence these responses.
METHODS: Fundic tone, gastric sensory responses and antral motility were evaluated in eight healthy volunteers after a probe with two sensors was placed in the antrum and a highly compliant balloon in the fundus. Isobaric balloon distentions were performed with a barostat. Study was repeated in six volunteers after intravenous atropine was given.
RESULTS: Fundic distention induced large amplitude antral contractions in all subjects. The area under the curve was higher (P<0.05) during fundic distention. First sensation was reported at 12±4 mmHg, moderate sensation at 18±4 mmHg and discomfort at 21±4 mmHg. Discomfort was associated with a decrease in antral motility. After atropine was given, the area under the curve of pressure waves and fundic tone decreased (P<0.05). Sensory thresholds were not affected.
CONCLUSIONS: Fundic balloon distention induces an antral motor response, the fundo-antral reflex, which in part may be mediated by cholinergic mechanisms.
- Citation: Rao SS, Kumar A, Harris B, Brown B, Schulze KS. Investigation of fundo-antral reflex in human beings. World J Gastroenterol 2005; 11(42): 6676-6680
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6676.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6676
The stomach performs several important functions. It subserves the function of accommodation and thereby acts as a storage organ. It also functions as a grinder that triturates food into smaller particles and as a pump that transports chyme into the small bowel in a controlled fashion[1]. These functions depend on a complex mix of neurohumoral mechanisms, visceral sensation, intrinsic reflexes, intragastric transport, nutrient composition, particulate size, and the coordinated motor activity of the gastroduodenal unit[2,3].
In ferrets, distension of the corpus produces phasic activity in the antrum, a response termed as excitatory corporo-antral reflex. This is probably mediated by cholinergic mechanisms and intramural gastric pathways[4,5]. Intramural excitatory and inhibitory reflexes have also been demonstrated in isolated gastric preparations[6-9]. Recent studies have suggested that balloon distention of the stomach may increase phasic activity in the antrum and duodenum[10,11]. Whether this phasic activity represents an intrinsic gastro-gastric reflex has not been well characterized.
Furthermore, previous studies have assessed some of the individual components of gastric function[12,13]; whereas an integrated assessment of the biomechanical and sensory properties of the stomach has been scarcely performed. Also, there is very little information regarding the integrated role of the stomach as a sensory, motor and reflex organ. Our hypothesis is that fundic balloon distention may induce reflex antral pressure activity and this response may be mediated by cholinergic mechanisms.
Our objectives were to examine the antral motor responses during step-wise balloon distentions of the fundus, to simultaneously assess the sensory and tone responses of the stomach and to examine if the sensory and motor effects were mediated by cholinergic mechanisms.
Eight healthy volunteers (m/f = 4/4) were recruited for this study. Their mean age was 32±4.95 years. None of them had a history of gastrointestinal or systemic ailments and none was using any medications. All had a normal physical examination. All participants gave written informed consent and the study protocol was approved by the Human Investigation Review Board of the University of Iowa College of Medicine.
A double lumen plastic-probe (6 mm in diameter) containing a 10 cm long, highly-compliant balloon was used (MUI Scientific; Toronto, ON, Canada). The capacity of the balloon was 600 mL. The balloon was connected to a barostat (GMB Distender II; G&J Electronics Inc., Toronto, Canada). The probe had two perfusion side holes, 5 and 8 cm from the distal end of the balloon. These holes were perfused with gas-free distilled water at a rate of 0.2 mL/min (15 psi) using a low-compliance pneumohydraulic perfusion system (Arndorfer Medical Specialties, Inc., Milwaukee, WI, USA) that connected to transducers (Medex Inc.; MX860-G8618, Hilliard, OH, USA). Intraluminal pressures were relayed to an analog data recorder/amplifier (Medtronics Polygraph, Medtronics Functional Diagnostics; MN, USA) and displayed on a computer monitor using a software program (Polygram for Windows; Synectics Medical AB). The balloon volume and pressure data from the barostat were fed to the polygraph via an interface (Golden Gate; G&J Electronics Inc.).
Thus, the computer display consisted of the intra-gastric balloon volume, intra-balloon pressure as well as the intraluminal pressure changes in the antrum. The manometric data and the ultrasound images were simultaneously fed into a digital splitter (American Dynamics Ao1479, Orangeburg, NY, USA).
The ultrasonographic image of the cross-sectional diameter of the gastric antrum allowed visualization of antral contractions and assurance that the probe was properly positioned (Acuson 128XP with a 3 mHz sector transducer). The digital splitter synchronized the two images and displayed these images on a monitor screen (VM-17; Javelin, Los Angeles, CA, USA) such that one half of the screen showed the combined manometry and barostat recording and the other half displayed the ultrasound image. These images were recorded on a VHS tape for future analysis.
After an overnight fast, the oropharynx was sprayed with a local anesthetic, pontocaine (Abbott Laboratories, North Chicago, IL, USA). Then the probe with the balloon was placed through the mouth into the stomach. The volunteers were asked to sit in a semi-recumbent position, such that the head end was elevated by 45°. The balloon was distended with 250 mL of air and the probe was slowly retracted until a “tug” was felt signaling that the proximal end of the balloon was located in the fundus. Subsequently, ultrasound images were obtained to check the probe location. The location of pressure sensors in the antrum was also confirmed by the occurrence of typical antral motor pattern consisting of 3-cycle/min activity. The balloon was deflated and the probe was anchored to the cheek with a tape.
After a rest period of 15 min, the balloon was distended by 1 mmHg increments to assess the minimum distending pressure, a pressure at which diaphragmatic oscillations are clearly visible[14]. The intraoperating pressure (IOP) was set at a value of 2 mmHg above the minimum distending pressure using previous criteria[9-11]. Subsequently, a baseline recording of intragastric tone was performed for a period of 20 min. Then isobaric balloon distentions were performed at 3 mmHg increments. Each distention was maintained for 8 min followed by a rest period of 8 min. Thirty seconds after each distention, the subject was asked to rate their sensation on a scale of 0-6 as published previously[14], 0 = no sensation, 1 = vague perception of mild sensation, 2 = definite perception of mild sensation, 3 = vague perception of moderate sensation, 4 = definite perception of moderate sensation, 5 = discomfort, and 6 = pain. If the subject reported discomfort at two incremental distentions or pain at any one distention, the balloon distentions were discontinued. Abdominal ultrasonography was performed intermittently to visualize the antral configuration and morphology. Blood pressure and heart rate were monitored throughout the study.
We administered intravenously 0.6 mg of atropine sulfate in six volunteers (4 m/2 f) after a rest period of 60 min. Five minutes after administration of atropine, the balloon distentions were repeated as described above. Ultrasound images were obtained once again to confirm the location of antral sensors.
Manometric recordings from the two antral pressure sensors were analyzed visually and manually with the assistance of Polygram for Windows software (Synectics Medical AB). Pressure waves that were ≥8 mmHg and ≥3 s in duration were included in the analysis. Artifacts were identified and excluded. There was good quality pressure activity at both channels in approximately 50% of the recordings and therefore an average of the pressure activity at the two antral channels was used. In the rest of the recordings, the pressure activity was more prominent in one of the antral channels and this channel was used for data analysis. The maximum amplitude, duration and area under the curve of each wave were calculated. We also measured the time interval between balloon distention and the onset of the first antral pressure wave as well as the total number of propagating pressure waves in the antrum during each inflation and deflation periods. Propagating waves were defined as pressure waves, which migrated across the antral leads within 6 s of each other and were categorized as either antegrade or retrograde depending on which of the two leads they first appeared in. A similar analysis of the antral pressure waves was performed after atropine injection.
Gastric tone was assessed by measuring the area under the curve of the gastric volume during isobaric balloon distentions as previously described[15-17]. The tone changes during and after distention were compared. Likewise, the tone changes obtained during the baseline study were compared with those after atropine injection.
During intragastric balloon distention, the minimum distending pressure that induced the first perception (a sensation of fullness and discomfort) were calculated. Likewise the sensory responses obtained after administration of atropine were compared to those obtained during the baseline study.
The data were presented as mean±SE. The number of pressure waves in the antrum during and after each balloon distention as well as before and after atropine injection was compared using Student’s t-test. The thresholds for sensory perception and the gastric tone changes before and after atropine injection were compared using ANOVA.
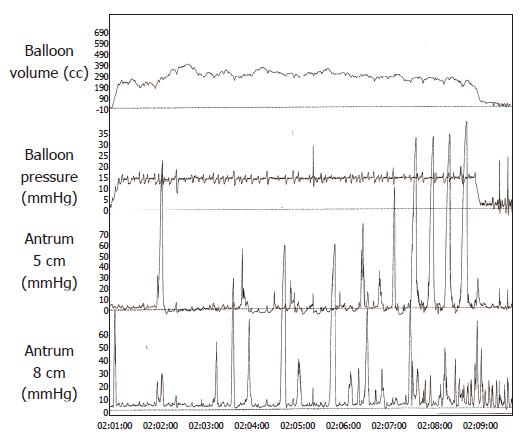
Fundic balloon distention induced antral pressure waves, typically with an amplitude of ≥50 mmHg (Figure 1). Occasionally, the distention-induced pressure activity persisted for several seconds even after the balloon was deflated, but in most instances the pressure activity ceased after deflation. Incremental balloon distention was associated with a steady increase in antral motility of up to 15 mmHg, but thereafter and particularly at 18 mmHg pressure there was a decrease in antral motility (Figure 2). Interestingly, during successive deflation periods, there was a trend towards progressive decrease in the area under the curve of pressure waves possibly reflecting a recovery of muscle tone.
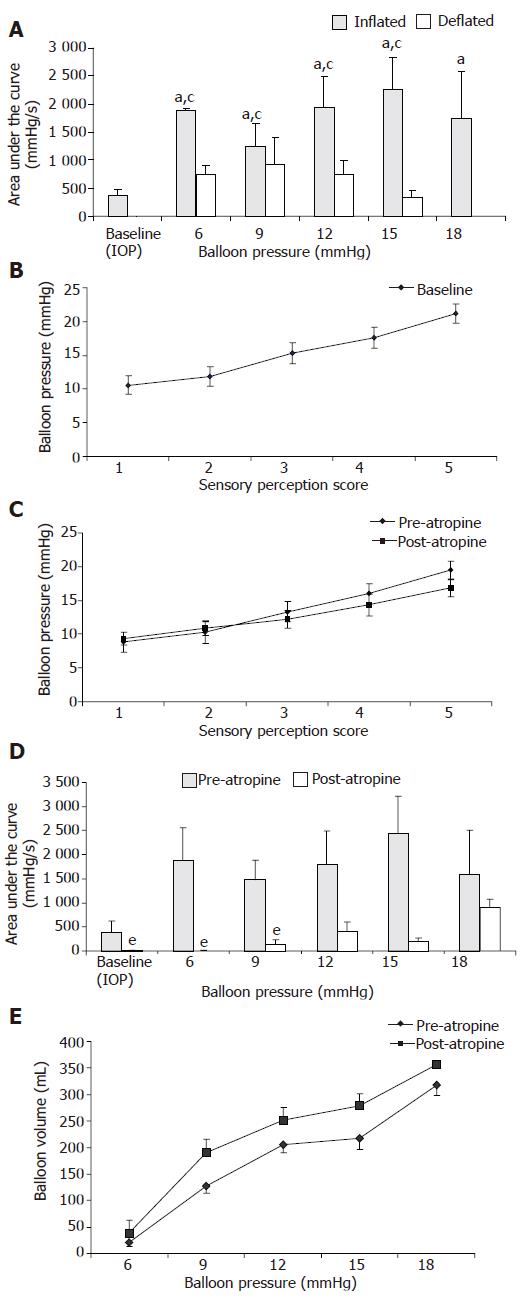
The mean amplitude of antral pressure waves was also higher (P<0.05) during balloon distention than during balloon deflation. For example at balloon distending pressures of 6, 12, and 15 mmHg, the amplitudes were (inflation vs deflation) 60(11) vs 42(9), 70(14) vs 33(7), and 65(11) vs 23(7) mmHg respectively. The area under the curve (AUC) of the pressure waves was also significantly higher (P<0.05) during balloon distention (Figure 2A).
The subjects reported a first sensation at distending pressures ranging from 6 to 15 mmHg, a definite per-ception between 6 and 18 mmHg, a vague perception of moderate sensation between 11 and 21 mmHg, a definite sensation of moderate fullness between 12 and 24 mmHg and definite discomfort between 15 and 28 mmHg (Figure 2B).
Visceral sensory responses The thresholds for first perception, fullness, and discomfort tended to be lower after administration of atropine but the difference was not significant (Figure 2C).
Antral pressure activity The area under the curve of pressure waves was significantly lower (P<0.05) after administration of atropine, particularly at balloon pressures of 6, 9, and 15 mmHg, but not at higher distending pressures (Figure 2D).
Fundic tone responses After administration of atropine, there was a significant increase (P<0.05) in balloon volume (Figure 2E) for the same corresponding level of intra balloon pressure, suggesting a decrease in fundic tone.
Cardiovascular responses The mean heart rate/min increased significantly (P<0.05) after administration of atropine during most of the distention except at 18 mmHg. There was no significant change in blood pressure.
We found that graded balloon distentions of the fundus induced antral pressure waves starting at thresholds that were not perceived by our healthy volunteers. This response was seen in all of our subjects. The area under the curve of pressure waves was significantly higher during the balloon inflation period than during the baseline period or the deflation period. Typically, the waves were ≥50 mmHg in amplitude. Ultrasound images confirmed that these pressure events were often lumen-occluding contractions. The contractions began within a few seconds after balloon distention. These features suggest the existence of an excitatory gastro-gastric reflex in human beings, wherein distention of the fundus induces antral contractions. In a previous uncontrolled pilot study, we showed that fundic balloon distention may induce antral and duodenal phasic activity[10]. However, in the previous study, gastric visceral sensation or tone was not assessed and likewise the possible role of cholinergic mechanism(s) was not explored.
One of the limitations of our study is that we recorded motility from only two antral pressure sensors and it is possible that some of the antral activities may have been missed. However, the number of distention-induced contractions and the area under the curve of pressure waves gradually increased to a distending pressure of 15 mmHg, but thereafter their incidence declined, suggesting that there appears to be some correlation of antral motor responses with the visceral sensory responses. All of our subjects tolerated balloon distentions up to a pressure of 15 mmHg above IOP. Beyond this level of distention, some subjects reported discomfort or pain which was associated with a decrease in pressure activity. Thus, it appears that fundic distention at either subthreshold levels of perception or at thresholds that produce first sensation or fullness may induce reflex antral contractions, whereas higher distending pressures that induce discomfort or pain may cause an attenuation of this response.
After administration of atropine, there was a significant increase in heart rate and decrease in the resting gastric tone. Furthermore, the antral motor activity was also significantly attenuated, particularly at lower levels of balloon distention. At higher distending pressures (≥12 mm Hg), some antral pressure activities were seen, though its incidence was lower than those observed before administration of atropine, suggesting that there is an adequate anti-cholinergic response and that the fundo-antral reflex may be partially mediated by cholinergic mechanisms[15]. However, we used a single dose of atropine and it is possible that over time there may have been a loss of anti-cholinergic effect. Also, there was no placebo arm, which is a limitation of this study. Nonetheless, these features suggest that either neuronal or drug-induced inhibition of cholinergic neurotransmission may partly affect gastric motor function. The sensory thresholds were either unchanged or somewhat decreased after administration of atropine, suggesting that gastric sensory responses may not be affected by cholinergic mechanisms, though the study was underpowered to assess this more completely.
Our study showed the possible existence of an intrinsic gastro-gastric reflex that can be induced by fundic distention. This response is different from the fundic relaxation that can be induced by antral distention[7,8] and appears to be partially mediated by cholinergic mechanisms and may in part be related to gastric sensation. Furthermore, unlike the Starling’s Law which states that distention of an intestinal segment is associated with proximal contraction and distal relaxation, our result shows that in the human stomach, distention of the fundus is not necessarily associated with antral relaxation. Whether this response plays a role in the trituration of food or in the transport of gastric contents remains to be examined. Also, whether an attenuated or absent fundo-antral reflex plays a role in the pathogenesis of diabetic gastroparesis or functional dyspepsia remains to be explored.
Supported in part by an American College of Gastroenterology Clinical Research Grant, RR00059 and by General Clinical Research Centers Program, R01DK57100-03, National Institutes of Health
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Rao SSC, Schulze-Delrieu K. The stomach, pylorus and duodenum. 2nd ed. London: Churchill Livingstone 1993; 373-392. [Cited in This Article: ] |
| 2. | Quigley EM. Gastric and small intestinal motility in health and disease. Gastroenterol Clin North Am. 1996;25:113-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Malagelada JR, Azpiroz F. Determinants of gastric emptying and transit in the small intestine. Bethesda (Maryland): American Physiological Society 1989; 909-937. [Cited in This Article: ] |
| 4. | Andrews PL, Grundy D, Scratcherd T. Reflex excitation of antral motility induced by gastric distension in the ferret. J Physiol. 1980;298:79-84. [PubMed] [Cited in This Article: ] |
| 5. | Grundy D, Hutson D, Scratcherd T. A permissive role for the vagus nerves in the genesis of antro-antral reflexes in the anaesthetized ferret. J Physiol. 1986;381:377-384. [PubMed] [Cited in This Article: ] |
| 6. | Hennig GW, Brookes SJ, Costa M. Excitatory and inhibitory motor reflexes in the isolated guinea-pig stomach. J Physiol. 1997;501:197-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Stadaas J, Aune S, Haffner JF. Effects of proximal gastric vagotomy on intragastric pressure and adaptation in pigs. Scand J Gastroenterol. 1974;9:479-485. [PubMed] [Cited in This Article: ] |
| 8. | Haffner JF, Stadaas J. Pressure responses to cholinergic and adrenergic agents in the fundus, corpus, and antrum of isolated rabbit stomachs. Acta Chir Scand. 1972;138:713-719. [PubMed] [Cited in This Article: ] |
| 9. | Leclere PG, Lefebvre RA. Investigation of the interaction between cholinergic and nitrergic neurotransmission in the pig gastric fundus. Br J Pharmacol. 1998;125:1779-1787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Rao SS, Vemuri S, Harris B, Schulze K. Fundic balloon distension stimulates antral and duodenal motility in man. Dig Dis Sci. 2002;47:1015-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Piessevaux H, Tack J, Geubel A, Janssens J. Influence of fundic distension on fasting antro-duodenal manometric patterns in man. Gastroenterology. 1998;114:G3374. [DOI] [Cited in This Article: ] |
| 12. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 771] [Cited by in F6Publishing: 734] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 13. | Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984;14:420-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 233] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Azpiroz F, Malagelada JR. Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterology. 1990;98:1193-1198. [PubMed] [Cited in This Article: ] |
| 15. | Azpiroz F, Malagelada JR. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology. 1987;92:934-943. [PubMed] [Cited in This Article: ] |
| 16. | Sarnelli G, Vos R, Cuomo R, Janssens J, Tack J. Reproducibility of gastric barostat studies in healthy controls and in dyspeptic patients. Am J Gastroenterol. 2001;96:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |