Summary
Abstract
Trastuzumab (Herceptin®) is a humanised monoclonal antibody used in the treatment of breast cancer that overexpresses human epidermal growth factor receptor 2 (HER2), which is associated with clinically aggressive disease and a poor prognosis.
The addition of intravenous trastuzumab to first-line chemotherapy improved the time to disease progression, objective response rate, duration of response, and overall survival in randomised, multicentre trials in women with HER2-positive metastatic breast cancer. As such, trastuzumab has become the standard of care in this setting, despite its high acquisition cost and potential for cardiac events, and is licensed for use in combination with paclitaxel (Europe and the US) or docetaxel (Europe). In addition, trastuzumab monotherapy is approved for use in patients with HER2-positive metastatic breast cancer who have previously received chemotherapy for their metastatic disease. Recent data from large phase III trials with trastuzumab in the adjuvant setting revealed significant improvements in disease-free and overall survival. Thus, trastuzumab is also rapidly becoming a standard component of adjuvant therapy for patients with HER2-positive early-stage breast cancer.
Pharmacological Properties
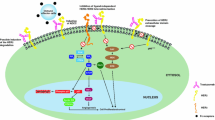
Trastuzumab is a humanised monoclonal antibody that binds selectively and with high affinity to the extracellular domain of HER2. Although the mechanism of its antitumour action has not been fully elucidated, trastuzumab promotes antibody-dependent cellular toxicity and the degradation of HER2 receptors, affects tumour growth and other signalling pathways, induces apoptosis and has antiangiogenic effects. Additive or synergistic effects have been demonstrated with trastuzumab and various cytotoxic drugs (e.g. paclitaxel, docetaxel, anthracyclines, cisplatin, carboplatin, vinorelbine, capecitabine) in HER2-overexpressing breast cancer cell lines and/or human breast tumour xenograft models.
Following a short-duration intravenous infusion of trastuzumab, peak serum concentrations are achieved after approximately 3–4 hours, and the drug is distributed into a volume approximately equal to that of serum. Although most clinical trials have used a once-weekly regimen of trastuzumab, population pharmacokinetic data based on a two-compartment model indicate that the elimination half-life is much longer than initially reported (28.5 vs ≈6 days), thus allowing for administration once every 3 weeks, as has been used in some recent clinical trials.
Therapeutic Efficacy
The addition of trastuzumab to paclitaxel therapy was associated with statistically and clinically significant improvements in the time to disease progression, objective response rate and duration of response, with little additional toxicity, compared with paclitaxel alone in a randomised phase III trial in women with HER2-positive (2+ or 3+ by immunohistochemistry [IHC]) metastatic breast cancer who had not previously received chemotherapy for their metastatic disease. In the same trial, which included two treatment arms of trastuzumab plus standard chemotherapy (paclitaxel or a combination of anthracycline plus cyclophosphamide) and two standard chemotherapy arms, a survival advantage was also demonstrated for trastuzumab plus chemotherapy versus chemotherapy alone. Subgroup and retrospective analyses from this and other trials in patients with HER2-positive breast cancer indicate that trastuzumab is effective only against those tumours with HER2 overexpression at the 3+ level (by IHC) or gene amplification (i.e. fluorescence in situ hybridisation [FISH]-positive).
In a subsequent randomised, multicentre trial in a more homogeneous group of patients (IHC 3+ or FISH-positive), the combination of trastuzumab plus docetaxel was associated with improvements in various parameters including time to disease progression and overall survival compared with docetaxel only. Various other combinations for first-line therapy in HER2-positive metastatic breast cancer have shown promising results in smaller phase II trials, and the use of trastuzumab monotherapy as second- or third-line treatment demonstrated beneficial effects in a large phase II trial.
Recent data from several large, phase III trials in patients with HER2-positive early-stage breast cancer have demonstrated that the addition of trastuzumab to chemotherapy in the adjuvant setting significantly improves disease-free survival. An interim combined analysis of two US Cooperative Group trials showed significant improvements in the primary endpoint of disease-free survival and the secondary endpoint of overall survival with trastuzumab-containing adjuvant therapy (an anthracycline-based regimen then paclitaxel plus trastuzumab) compared with adjuvant therapy comprising an anthracycline-based regimen followed by paclitaxel. Across four phase III trials (NCCTG N9831, NSABP B-31, HERA and BCIRG 006), interim/preliminary results indicate that trastuzumab-containing adjuvant therapy was associated with a 39–52% reduction in the risk of cancer recurrence compared with chemotherapy-only adjuvant regimens in patients with HER2-positive (IHC 3+ and/or FISH-positive) early-stage breast cancer.
Tolerability
The most common adverse events associated with trastuzumab are infusion-related symptoms, such as fever and chills, usually occurring during administration of the first dose. In pivotal trials in which women with metastatic breast cancer received trastuzumab as monotherapy or in combination with paclitaxel, the following adverse events were attributed to trastuzumab and were reported in ≥10% of patients: abdominal pain, asthenia, chest pain, chills, fever, headache, pain, diarrhoea, nausea, vomiting, arthralgia, myalgia and rash.
Trastuzumab is also associated with a number of serious adverse events including cardiac events, severe hypersensitivity reactions (including anaphylaxis), infusion-related reactions and pulmonary events. The risk of ventricular dysfunction and congestive heart failure in patients treated with trastuzumab alone or in combination with paclitaxel or docetaxel is particularly increased if administered in combination with anthracycline-containing chemotherapy. Patients should undergo a thorough cardiac assessment prior to beginning trastuzumab therapy, and cardiac function should be monitored during trastuzumab therapy. Trastuzumab can also increase chemotherapy-induced neutropenia.









Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Imaginis. Breast Cancer: statistics on incidence, survival, and screening [online]. Available from URL: http://imaginis.com/breasthealth/statistics.asp [Accessed 2005 Dec 8]
Tyczynski JE, Bray F, Parkin DM. Breast cancer in Europe. ENCR Cancer fact sheets 2002 Dec; Vol. 2 [online]. Available from URL: http://www.encr.com.fr/breast-factsheets.pdf [Accessed 2005 Dec 7]
American Cancer Society. What are the key statistics for breast cancer? [online]. Available from URL: http://www.cancer.org [Accessed 2001 Sep 8]
American Cancer Society. Breast cancer: treatment guidelines for patients (version VII/August 2005) [online]. Available from URL: http://www.cancer.org [Accessed 2005 Sep 9]
Akiyama T, Sudo C, Ogawara H, et al. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986 Jun 27; 232(4758): 1644–6
Harries M, Smith I. The development and clinical use of trastuzumab (Herceptin). Endocr Relat Cancer 2002 Jun; 9(2): 75–85
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989 May 12; 244(4905): 702–12
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987 Jan 9; 235(4785): 177–82
Roche Products Ltd.. Herceptin®: summary of product characteristics (Annex 1); 2000 Aug 28 [online]. Available from URL: http://www.emea.eu.int/humandocs/PDFs/EPAR/Herceptin/H-278-PI-en.pdf [Accessed 2005 Nov 18]
Pauletti G, Godolphin W, Press MF, et al. Detection and quantitation of HER-2/neu gene amplification in human breast cancer archival material using fluorescence in situ hybridization. Oncogene 1996 Jul 4; 13(1): 63–72
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992 May; 89: 4285–9
McKeage K, Perry CM. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER 2. Drugs 2002; 62(1): 209–43
Herceptin® (trastuzumab) prescribing information. San Francisco (CA): Genentech Inc. 2005 Feb
Albaneil J, Codony J, Rovira A, et al. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C 4. Adv Exp Med Biol 2003; 532: 253–68
Baselga J, Norton L, Albaneil J, et al. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–31
Pietras RJ, Pegram MD, Finn RS, et al. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998; 17: 2235–49
Pegram MD, Konecny GE, O’Callaghan C, et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004 May 19; 96(10): 739–49
Herceptin® product monograph, 2004 update. Basel: F. Hoffmann-La Roche Ltd, 2004
Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol 2005 Apr 10; 23(11): 2460–8
Izumi Y, Xu L, di Tomaso E, et al. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 2002; 416: 279–80
Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER 2. Clin Cancer Res 2004 Sep 1; 10(17): 5650–5
Fujimoto-Ouchi K, Sekiguchi F, Tanaka Y. Antitumor activity of combinations of anti-HER-2 antibody trastuzumab and oral fluoropyrimidines capecitabine/5′-dFUrd in human breast cancer models. Cancer Chemother Pharmacol 2002 Mar; 49(3): 211–6
Liang K, Lu Y, Jin W, et al. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2003 Nov; 2(11): 1113–20
Argiris A, Wang CX, Whalen SG, et al. Synergistic interactions between tamoxifen and trastuzumab (Herceptin). Clin Cancer Res 2004 Feb 15; 10(4): 1409–20
Ropero S, Menendez JA, Vazquez-Martin A, et al. Trastuzumab plus tamoxifen: anti-proliferative and molecular interactions in breast carcinoma. Breast Cancer Res Treat 2004 Jul; 86(2): 125–37
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17(9): 2639–48
Baselga J, Carbonell X, Castaneda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 2005 Apr 1; 23(10): 2162–71
Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 2003 Nov 1; 21(21): 3965–71
Harris K, Washington CB, Lieberman J-F, et al. A population pharmacokinetic (PK) model for trastuzumab (Herceptin) and implications for clinical dosing [abstract no. 488]. Proc Am Soc Clin Oncol 2002; 21 Suppl. Pt 1: 123a
Nieto Y, Vredenburgh JJ, Shpall EJ, et al. Phase II feasibility and pharmacokinetic study of concurrent administration of trastuzumab and high-dose chemotherapy in advanced HER2+ breast cancer. Clin Cancer Res 2004 Nov 1; 10: 7136–43
Czejka M, Ostermann E, Muric L, et al. Pharmacokinetics of gemcitabine combined with trastuzumab in patients with advanced breast cancer. Onkologie 2005 Jun; 28(6–7): 318–22
Lunardi G, Vannozzi MO, Bighin C, et al. Influence of trastuzumab on epirubicin pharmacokinetics in metastatic breast cancer patients. Ann Oncol 2003 Aug; 14(8): 1222–6
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER 2. N Engl J Med 2001 Mar 15; 344(11): 783–92
Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol 2005 Jul 1; 23(19): 4265–74
Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol 2003 Jan; 21(1): 46–53
Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002 Feb 1; 20(3): 719–26
Mass RD, Press MF, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 2005 Aug; 6(3): 240–6
Silverstein MJ. Recent advances: diagnosis and treatment of early breast cancer. BMJ 1997 Jun 14; 314(7096): 1736–9
Buehler H, Bangemann N, Evers K, et al. Effective HER-2/neu diagnosis in breast cancer by a combination of immunohistochemistry and FISH [abstract no. 294]. 36th Annual Meeting of the American Society of Clinical Oncology; 2000 May 20–23; New Orleans (LA), 76a
Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 2004 Mar 15; 22(6): 1063–70
Burris H, Yardley D, Jones S, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol 2004 May 1; 22(9): 1621–9
Brufsky A, Orlando M, Fox K, et al. Phase II study of gemcitabine and trastuzumab combination therapy in patients with HER2-overexpressing metastatic breast cancer: first stage results [abstract no. 3047]. Breast Cancer Res Treat 2004; 88 Suppl. 1: S 128. Plus poster presented at the 27th San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Tusquets I, Ramos M, Gil M, et al. Study of docetaxel and trastuzumab combination, administered every 21 days, in patients with metastatic breast cancer and HER-2 over-expression [abstract no. 3054]. Breast Cancer Res Treat 2004; 88 Suppl. 1: 130–1. Plus poster presented at the 27th Annual San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Perez EA, Rowland KM, Suman VJ, et al. N98-32-52: efficacy and tolerability of two schedules of paclitaxel, carboplatin and trastuzumab in women with HER2 positive metastatic breast cancer: a North Central Cancer Treatment Group randomized phase II trial [abstract no. 216]. 26th Annual San Antonio Breast Cancer Symposium; 2003 Dec 3–6; San Antonio (TX) [online]. Available from URL: http://www.sabcs.org [Accessed 2004 Mar 8]
Arteaga CL, O’Neil A, Moulder SL, et al. ECOG1100: a phase I–II study of combined blockade of the erbB receptor network with trastuzmab and gefitinib (Iressa) in patients with HER2-overexpressing metastatic breast cancer [abstract no. 25]. Breast Cancer Res Treat 2004; 88 Suppl. 1: 15–6
Xu L, Song S, Zhu J, et al. Results of a phase II trial of Herceptin® plus Xeloda® in patients with previously untreated HER2-positive metastatic breast cancer [abstract no. 3049]. Breast Cancer Res Treat 2004; 88 Suppl. 1: 128–9. Plus poster presented at the 27th Annual San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 2002 Apr 1; 20(7): 1800–8
Montemurro F, Choa G, Faggiuolo R, et al. Safety and activity of docetaxel and trastuzumab in HER2 overexpressing metastatic breast cancer: a pilot phase II study. Am J Clin Oncol 2003 Feb; 26(1): 95–7
Tedesco KL, Thor AD, Johnson DH, et al. Docetaxel combined with trastuzumab is an active regimen in HER-2 3+ overexpressing and fluorescent in situ hybridization-positive metastatic breast cancer: a multi-institutional phase II trial. J Clin Oncol 2004 Mar 15; 22(6): 1071–7
Brufsky AM, Cleary D, Fuchs C, et al. First-line chemotherapy for metastatic breast cancer (MBC) with docetaxel (T), carboplatin (C), and traztuzumab (H): a phase II trial [abstract no. 71]. 39th Annual Meeting of the American Society of Clinical Oncology; 2003 May 31–June 3; Chicago (IL), 18
Pegram MD, Pienkowski T, Northfelt DW, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst 2004 May 19; 96(10): 759–69
Slamon D, Yeon CH, Pienkowski T, et al. Survival analysis from two open-label non-randomized phase II trials of trastuzumab combined with docetaxel and platinums (C, cisplatin or carboplatin) in women with HER2+ advanced breast cancer [abstract no. 642 plus poster]. 40th Annual Meeting of the American Society of Clinical Oncology; 2004 Jun 5–8; New Orleans (LA)
Pienkowski T, Fumoleau P, Eiermann W, et al. Taxotere, cisplatin and Herceptin (TCH) in first-line HER2 positive metastatic breast cancer (MBC) patients, a phase II pilot study by the Breast Cancer International Research Group (BCIRG 101) [abstract no. 2030]. 37th Annual Meeting of the American Society of Clinical Oncology; 2001 May 12–15; San Francisco (CA)
Yardley D, Greco A, Porter LL, et al. First-line treatment of HER2-positive metastatic breast cancer with trastuzumab, docetaxel and vinorelbine: results of a Minnie Pearl Cancer Research Network trial [abstract no. 146P]. Ann Oncol 2004; 15 Suppl. 3: iii 39. Plus poster presented at the 29th European Society of Medical Oncology Congress; 2004 Oct 29–Nov 2; Vienna
Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, et al. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer: a Hellenic Cooperative Oncology Group phase II study. Ann Oncol 2001 Nov; 12: 1545–51
Gasparini G, Morabito A, De Sio L, et al. Preliminary clinical results of a randomized phase IIb study of weekly pacltiaxel +/− trastuzumab as first-line therapy of patients with HER-2/neu positive metastatic breast cancer [abstract no. 227]. 26th Annual San Antonio Breast Cancer Symposium; 2003 Dec 3–6; San Antonio (TX) [online]. Available from URL: http://www.sabcs.org [Accessed 2003 Dec 3]
John M, Hindenburg HJ, Hinke A, et al. Weekly paclitaxel plus trastuzumab in metastatic breast cancer: a multicentre German trial [abstract no. 458]. EJC Supplements 2003 Sep; 1(5): S139–40
Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol 2001; 19(10): 2587–95
Cortes J, Climent M, Lluch A, et al. Updated results of a phase II study (M77035) of Myocet® combined with weekly Herceptin® and paclitaxel in patients with HER2-positive locally advanced or metastatic breast cancer [abstract no. 3041]. Breast Cancer Res Treat 2004; 88 Suppl. 1: 125–6
Bianchi G, Albaneil J, Eiermann W, et al. Pilot trial of trastuzumab starting with or after the doxorubicin component of a doxorubicin plus paclitaxel regimen for women with HER2-positive advanced breast cancer. Clin Cancer Res 2003 Dec 1; 9 (16 Pt 1): 5944–51
Colomer R, Mayordomo JI, Calvo L, et al. HER2 ECD-positive metastatic breast cancer treated with gemcitabine, paclitaxel plus trastuzumab [abstract no. 3046]. Breast Cancer Res Treat 2004; 88 Suppl. 1: S127-8. Plus poster presented at the 27th Annual San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Fountzilas G, Christodoulou C, Tsavdaridis D, et al. Paclitaxel and gemcitabine, as first-line chemotherapy, combined with trastuzumab in patients with advanced breast cancer: a phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG). Cancer Invest 2004; 22(5): 655–62
Miller KD, Sisk J, Gize G, et al. Phase II study of gemcitabine, paclitaxel and trastuzumab in metastatic breast cancer: a Hoosier Oncology Group trial [abstract no. 437]. 25th Annual San Antonio Breast Cancer Symposium; 12 Dec 2002; San Antonio (TX) [online]. Available from URL: http://www.sabcs.org [Accessed 2003 Feb 10]
Bayo-Calero JL, Mayordomo-Camara JI, Sanchez-Rovira P, et al. A multicenter study with trastuzumab and vinorelbine as first and 2nd line treatment in patients with Her2 positive metastatic breast cancer [abstract no. 5069]. Breast Cancer Res Treat 2004; 88 Suppl. 1: 207–8
Burstein HJ, Harris LN, Marcom PK, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol 2003 Aug 1; 21(15): 2889–95
Chan A, Petruzelka L, Untch M, et al. Long term survival of vinorelbine and trastuzumab as first line therapy for HER2-positive metastatic breast cancer patients [abstract no. 587]. J Clin Oncol 2005 Jun 1; 23 (16 Suppl.): 25s. Plus poster presented at the 41st Annual Meeting of the American Society of Clinical Oncology; 2005 May 13–17; Orlando (FL)
de Wit M, Becker K, Thomssen C, et al. Vinorelbine and trastuzumab as first line therapy in patients with HER2-positive metastatic breast cancer: interim analysis of a prospective, open-label, multicentre phase II trial [abstract no. 138P]. Ann Oncol 2004; 15 Suppl. 3: iii37
Jahanzeb M, Mortimer JE, Yunus F, et al. Phase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancer. Oncologist 2002; 7(5): 410–7
Verma S, Leyland-Jones B, Ayoub J-P, et al. Efficacy and safety of three-weekly herceptin with paclitaxel in women with Her2-positive metastatic breast cancer: preliminary results of a phase II trial [abstract no. 538]. Eur J Cancer 2001 Oct 21; 37 Suppl. 6: S146
Scholz U, Luck HJ, Schippert C, et al. Trastuzumab (Herceptin) combined with weekly paclitaxel in the treatment of metastatic breast cancer: a phase II study [abstract no. 27]. Breast Cancer Res Treat 2000; 64: 122
Bangemann N, Kuhle A, Willrodt RG, et al. Treatment of HER2 overexpressing metastatic breast cancer with trastuzumab (Herceptin) and chemotherapy [abstract no. 530]. Breast Cancer Res Treat 2000; 64: 123
Gori S, Colozza M, Mosconi AM, et al. Phase II study of weekly paclitaxel and trastuzumab in anthracycline- and taxane-pretreated patients with HER2-overexpressing metastatic breast cancer. Br J Cancer 2004 Jan 12; 90(1): 36–40
Yamamoto D, Iwase S, Kitamura K, et al. Multicenter phase II study of trastuzumab and capecitabine as first- or second-line treatment in HER2 over-expressing metastatic breast cancer (Japan Breast Cancer Study Group: JBCSG-003) [abstract no. 802]. J Clin Oncol 2005 Jun 1; 23 (16 Pt 1 Suppl.): 78s
Bauer-Kosinska B, Lemanska I, Glogowska I, et al. Efficacy and toxicity of docetaxel or cisplatin chemotherapy in combination with trastuzumab in the treatment of patients with chemotherapy pre-treated HER-2/neu overexpressed metastatic breast cancer [abstract no. 464]. EJC Supplements 2003 Sep; 1(5): S141
Peacock NW, Bearden J, Schnell F, et al. Phase II trial of gemcitabine plus trastuzumab in minimally pretreated HER2 overexpressing metastatic breast cancer [abstract no. 704]. J Clin Oncol 2005 Jun 1; 23 (16 Pt 1 Suppl.): 54s
Christodoulou C, Fountzilas G, Razi E, et al. Gemcitabine and trastuzumab combination as salvage treatment in patients with HER 2-positive metastatic breast cancer [abstract no. 166]. 39th Annual Meeting of the American Society of Clinical Oncology; 2003 May 31–Jun 3; Chicago (IL)
O’Shaughnessy J, Vukelja SJ, Marsland T, et al. Phase II trial of gemcitabine plus trastuzumab in metastatic breast cancer patients previously treated with chemotherapy: preliminary results. Clin Breast Cancer 2002 May; 3 Suppl. 1: 17–20
Burstein HJ, Kuter I, Campos SM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol 2001; 19(10): 2722–30
Papaldo P, Fabi A, Pino MS, et al. Comparison between vinorelbine in HER2-negative and vinorelbine plus trastuzumab in HER2-positive pretreated metastatic breast cancer patients [abstract no. 296]. 39th Annual Meeting of the American Society of Clinical Oncology; 2003 May 31–Jun 3; Chicago (IL)
Gebbia V, Chiarenza M, Aiello R, et al. Trastuzumab in combination with i.v. vinorelbine in advanced breast cancer patients with HER2 overexpression [abstract no. 137P]. Ann Oncol 2004; 15 Suppl. 3: 37
Suzuki Y, Tokuda Y, Saito Y, et al. Combination of trastuzumab and vinorelbine in metastatic breast cancer. Jpn J Clin Oncol 2003 Oct; 33(10): 514–7
Montemurro F, Faggiuolo R, Redana S, et al. Continuation of trastuzumab beyond disease progression [letter]. J Clin Oncol 2005 Apr 20; 23(12): 2866–8; discussion 2868-9
Research to practice: HER2-positive disease [poster]. 27th Annual San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 2004 Apr; 5(1): 52–8; discussion 59-62
Fountzilas G, Razis E, Tsavdaridis D, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the Hellenic Cooperative Oncology Group. Clin Breast Cancer 2003 Jun; 4(2): 120–5
Stemmler H-J, Kahlert S, Siekiera W, et al. Prolonged survival of patients receiving trastuzumab beyond disease progression for HER2 overexpressing metastatic breast cancer (MBC). Ontologie 2005; 28: 582–6
TBP study with capecitabine plus minus trastuzumab [online]. Available from URL: http://www.clinicaltrials.gov/ct/gui/show/NCT00148876 [Accessed 2005 Nov 21]
Clemens M, von Minckwitz G, Loehr A, et al. Single-agent herceptin in patients with pretreated HER2-positive metastatic breast cancer: reponse, survival and tolerability. Ann Oncol 2002; 13 Suppl. 5: 50
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005 Oct 20; 353(16): 1659–72
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005 Oct 20; 353(16): 1673–84
Slamon D, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study [abstract no. 1 plus oral presentation]. 28th Annual San Antonio Breast Cancer Symposium; 2005 Dec 8–11; San Antonio (TX)
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 2005 Jun 1; 23(16): 3676–85
Coudert BP, Arnould L, Moreau L, et al. Pathological complete response rate with neoadjuvant trastuzumab and docetaxel chemotherapy in HER-2 positive (3+) locally advanced breast cancer [abstract no. 128P]. Ann Oncol 2004; 15 Suppl. 3: 34. Plus poster presented at the 29th European Society of Oncology Congress; 2004 Oct 29–Nov 2; Vienna
Van Pelt AE, Mohsin S, Elledge RM, et al. Neoadjuvant trastuzumab and docetaxel in breast cancer: preliminary results. Clin Breast Cancer 2003 Dec; 4(5): 348–53
Bines J, Murad A, Lago S, et al. Primary treatment with weekly docetaxel (Taxotere) and trastuzumab (Herceptin) for HER-2 overexpressing locally advanced breast cancer [abstract no. 370]. EJC Supplements 2003; 1 (5 Suppl.): S114
Harris LN, Burstein H, Gelman R, et al. Preoperative trastuzumab and vinorelbine is a well-tolerated, active regimen for Her2 3+/FISH+ stage II/III breast cancer [abstract no. 397]. EJC Supplements 2003; 1(5): S121
Hurley J, Doliny P, Silva O, et al. Neoadjuvant herceptin/taxotere/cisplatin in the treatment of locally advanced and inflammatory breast cancer [abstract no. 196]. 38th Annual Meeting of the American Society of Clinical Oncology; 2002 May 18–21; Orlando (FL)
Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003 Jun 15; 97(12): 2972–7
Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 2004 Aug 16; 91(4): 639–43
Lower EE, Drosick DR, Blau R, et al. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer 2003 Jun; 4(2): 114–9
Yau T. Brain as a sanctuary site for early relapse in patients with advanced breast cancer treated with trastuzumab [abstract no. P101]. Breast 2005 Feb; 14 Suppl. 1: S43
Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 2004 Aug 15; 101(4): 810–6
Montemurro F, Aglietta M. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma [letter]. Cancer 2005 Mar 15; 103(6): 1314–5; author reply 1315
Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005 Nov 1; 23(31): 7811–9
MedWatch — 2005 safety information alerts: Herceptin (trastuzumab) [online]. Available from URL: http://www.fda.gov/medwatch/safety/2005/safety05.htm [Accessed 2005 Sep 9]
Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer 2002 Oct 1; 95(7): 1592–600
Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J Clin Oncol 2004 Jan 15; 22(2): 322–9
Klein PM, Dybdal N. Trastuzumab and cardiac dysfunction: update on preclinical studies. Semin Oncol 2003 Oct; 30 (5 Suppl. 16): 49–53
Schneider JW, Chang AY, Garratt A. Trastuzumab cardiotoxicity: speculations regarding pathophysiology and targets for further study. Semin Oncol 2002 Jun; 29 (3 Suppl. 11): 22–8
Schneider JW, Chang AY, Rocco TP. Cardiotoxicity in signal transduction therapeutics: erhB2 antibodies and the heart. Semin Oncol 2001 Oct; 28 (5 Suppl. 16): 18–26
Behr TM, Behe M, Wormann B. Trastuzumab and breast cancer. N Engl J Med 2001; 345(13): 995–6
British National Formulary. 49 ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2005
Fleming T, editor. Red book: pharmacy’s fundamental reference. 2005 edition. Stamford (CT): Thomson Healthcare, 2005
Norum J, Risberg T, Olsen JA. A monoclonal antibody against HER-2 (trastuzumab) for metastatic breast cancer: a model-based cost-effectiveness analysis. Ann Oncol 2005; 16: 909–14
Neyt MJ, Albrecht JA, Clarysse B, et al. Cost-effectiveness of Herceptin®: a standard cost model for breast-cancer treatment in a Belgian university hospital. Int J Technol Assess Health Care 2005; 21(1): 132–7
Elkin EB, Weinstein MC, Winer EP, et al. HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 2004 Mar 1; 22(5): 854–63
Hornberger J, Kerrigan M, Foutel V. Cost-effectiveness of trastuzumab (Herceptin®) for treatment of metastatic breast cancer [abstract no. 185P]. Ann Oncol 2002; 13 Suppl. 5: 52
Miadi-Fargier H, Trillet-Lenoir V, Ganne C, et al. Impact of innovative and expensive therapies in the treatment of metastatic breast cancer (MBC): focus on trastuzumab (Herceptin (H)) [abstract no. PCN12]. Value Health 2002; 5: 541
National Institute for Clinical Excellence. Guidance on the use of trastuzumab for the treatment of advanced breast cancer: technology appraisal guidance, No. 34. 2002 Mar
Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med 2005 Oct 20; 353(16): 1734–6
Mayor S. Women with early breast cancer to be tested for trastuzumab treatment [news]. BMJ 2005 Oct 15; 331: 864
Hortobagyi GN. Overview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer. Semin Oncol 2001 Dec; 28 (6 Suppl. 18): 43–7
Spicer J, Harries M, Ellis P. Adjuvant trastuzumab for HER2-positive breast cancer [correspondence]. Lancet 2005 Aug 20; 366: 634
Smith M. Oh, Canada: public outcry pushed demand for trastuzumab for early-stage breast cancer. J Natl Cancer Inst 2005 Sep 21; 97(18): 1327
Priest L. New cancer drug limited to few: impressive in cutting disease recurrence, Herceptin still lacks government funding [online]. Available from URL: http://www.theglobeandmail.com [Accessed 2005 Jun 8]
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: N. Bangemann, Medical Center Benjamin Franklin, Free University, Berlin, Germany; E.B. Elkin, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, USA; I.O. Ellis, Department of Histopathology, Nottingham City Hospital, Nottingham, England; G. Fountzilas, 1st Department of Internal Medicine, Oncology Section, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece; J. Horton, Comprehensive Breast Cancer Program, H. Lee Moffitt Cancer Center & Research Institute, Tampa, Florida, USA; B. Leyland-Jones, Department of Oncology, McGill University, Montreal, Quebec, Canada; F. Montemurro, Unit of Medical Oncology, Institute for Cancer Research and Treatment, Torino, Italy.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘trastuzumab’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE search terms were ‘trastuzumab’ and ‘breast cancer’. EMBASE search terms were ‘trastuzumab’ and ‘breast cancer’. AdisBase search terms were ‘trastuzumab’ and ‘breast cancer’. Searches were last updated 8 February 2006.
Selection: Studies in patients with HER2-positive breast cancer who received trastuzumab. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Trastuzumab, metastatic breast cancer, early-stage breast cancer, adjuvant therapy, neoadjuvant therapy, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, pharmacoeconomics.
Rights and permissions
About this article
Cite this article
Plosker, G.L., Keam, S.J. Trastuzumab. Drugs 66, 449–475 (2006). https://doi.org/10.2165/00003495-200666040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666040-00005