Abstract
Objectives: The aims of this study were to investigate the safety, tolerability, pharmacokinetics and pharmacodynamics of regadenoson (CVT-3146) in healthy, male volunteers.
Methods: Thirty-six healthy, male volunteers aged 18–50 years were included in this randomised, double-blind, crossover, placebo-controlled study to evaluate single intravenous bolus doses of regadenoson that ranged from 0.1 to 30.0 μg/kg. Subjects received one dose of regadenoson or placebo on successive days while supine, then the same dose of regadenoson or placebo on successive days while standing. As part of the safety evaluation, vital signs and adverse events were monitored and recorded throughout the course of the study in all subjects. Up to 20 plasma samples were collected for regadenoson concentration determination within the 24 hours after each supine dosage. All urine was collected during the 24-hour time period post-dose and an aliquot was used for the determination of the regadenoson concentration. Heart rate and blood pressure were recorded at many of the same timepoints that the samples for the pharmacokinetic analysis were taken. A non linear mixed-effect modelling approach, using the software NONMEM, was utilised in modelling the plasma and urine concentration-time profiles and temporal changes in heart rate after regadenoson administration in the supine position. The influences of several covariates, including bodyweight, body mass index and age, on pharmacokinetic model parameters were investigated.
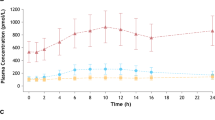
Results: Adverse events were more prevalent at regadenoson doses above 3 μg/kg, and the increase in the occurrence of adverse events was dose-related. Most of the adverse events were related to vasodilation and an increase in heart rate and were generally of mild to moderate severity. Based on the severity and frequency of adverse events, the maximum tolerated doses of regadenoson were deemed to be 10 μg/kg in the standing position and 20 μg/kg in the supine position. The pharmacokinetics of regadenoson were successfully described by a three-compartment model with linear clearance. Following intravenous bolus dose administration, regadenoson was rapidly distributed throughout the body, followed by relatively slower elimination (terminal elimination half-life of approximately 2 hours). The clearance was estimated to be 37.8 L/h, with renal excretion accounting for approximately 58% of the total elimination. The volume of distribution of the central compartment and the volume of distribution at steady state were estimated to be 11.5L and 78.7L, respectively. Individual pharmacokinetic parameter estimates were fixed in the pharmacodynamic model, where changes in heart rate were related to plasma drug concentrations using a Michaelis-Menten model. The maximum heart rate increase (Emax) and plasma regadenoson concentration causing a 50% increase in the maximum heart rate (EC50) were estimated to be 76 beats per minute and 12.3 ng/mL, respectively. None of the tested covariates was found to be correlated with any of the pharmacokinetic model parameters.
Conclusions: The pharmacokinetics and the effects of regadenoson on heart rate were successfully described using pharmacokinetic/pharmacodynamic modelling. The lack of a correlation between the model estimates and various baseline patient demographics supports unit-based dose administration of regadenoson.











Similar content being viewed by others
References
Ranhosky A, Kempthorne-Rawson J. The safety of intravenous dipyridamole thallium myocardial perfusion imaging. Intravenous Dipyridamole Thallium Imaging Study Group. Circulation 1990; 81(4): 1205–9
Cerqueira MD, Verani MS, Schwaiger M, et al. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol 1994; 23(2): 384–9
Trochu JN, Zhao G, Post H, et al. Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J Cardiovasc Pharmacol 2003; 41(1): 132–9
Zhao G, Linke A, Xu X, et al. Comparative profile of vasodilation by CVT-3146, a novel A2A receptor agonist, and adenosine in conscious dogs. J Pharmacol Exp Ther 2003; 307(1): 182–9
Ellenbogen KA, O’Neill G, Prystowsky EN, et al. Trial to evaluate the management of paroxysmal supraventricular tachycardia during an electrophysiology study with tecadenoson. Circulation 2005; 111(24): 3202–8
Meade CJ, Dumont I, Worrall L. Why do asthmatic subjects respond so strongly to inhaled adenosine? Life Sci 2001; 69(11): 1225–40
Jonsson EN, Karlsson MO. Xpose- an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 1999; 58: 51–64
Holford N. The visual predictive check: superiority to standard diagnostic (Rorschach) plots [abstract No. 738]. 14th annual meeting of PAGE (Population Approach Group Europe); 2005 Jun 16–17; Pamplona
Jusko WJ, Levy G. Pharmacokinetic evidence for saturable renal tubular reabsorption of riboflavin. J Pharm Sci 1970; 59: 765–72
Shryock JC, Snowdy S, Baraldi PG, et al. A2A-adenosine receptor reserve for coronary vasodilation. Circulation 1998; 98(7): 711–8
Engelstein ED, Lerman BB, Somers VK, et al. Role of arterial chemoreceptors in mediating the effects of endogenous adenosine on sympathetic nerve activity. Circulation 1994; 90(6): 2919–26
Dhalla AK, Wong MY, Wang WQ, et al. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J Pharmacol Exp Ther 2006 Feb; 316(2): 695–702
Acknowledgements
This study was funded by CV Therapeutics, Inc. Dr Gordi and Dr Wolff are former employees and current consultants to CV Therapeutics Inc. Dr Lieu and Dr Frohna are former employees and Dr Ling is a current employee of CV Therapeutics Inc.
The authors would like to thank Dr Mats Karlsson (Uppsala University) for providing the NONMEM coding for integration of urine data in the pharmacokinetic model.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gordi, T., Frohna, P., Sun, HL. et al. A Population Pharmacokinetic/Pharmacodynamic Analysis of Regadenoson, an Adenosine A2A-Receptor Agonist, in Healthy Male Volunteers. Clin Pharmacokinet 45, 1201–1212 (2006). https://doi.org/10.2165/00003088-200645120-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645120-00005