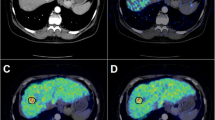
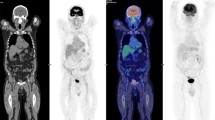
Abstract
Objectives
The purpose of this study was to evaluate the adverse effects of chronic marked hyperglycemia on clinical diagnostic performance of positron emission tomography (PET) using 18F-fuorodeoxyglucose (FDG).
Methods
Fifty-seven scans of 54 patients, who received FDG-PET for the diagnosis of various cancer(s), and who showed high plasma glucose level of more than 200 mg/dl at the time of administration of FDG in spite of at least 4-h fasting, were retrospectively analyzed. In the clinical follow-up, this high plasma glucose was confirmed as chronic hyperglycemia derived from uncontrolled diabetes (n = 32) and untreated diabetes (n = 25). Based on the final diagnosis of malignancy obtained by histopathology or clinical follow-up for at least 6 months, the diagnostic performance of visual PET analysis was evaluated.
Results
Excluding nine scans of nine patients without sufficient follow-up, final diagnosis was obtained in 48 scans of 45 patients. In 36 scans of 36 patients, at least one malignant lesion was finally confirmed, and true-positive and false-negative results were obtained in 30 and six cases, respectively. Six cases showed false-negative results due to low FDG-avid pathological characteristics (hepatocellular carcinoma, etc.), chemotherapeutic effect or small tumor size. Overall, the patient-based sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy were 83, 83, 94, 63 and 83%, respectively. In lesion-based diagnosis, 56 of 75 lesions (74%) were depicted by PET, while 19 lesions were negative on PET, also due to low FDG-avid characteristics or small size (less than 15 mm).
Conclusions
At the time of chronic hyperglycemia (not acute hyperglycemia), the adverse effect caused by high plasma glucose level was minimum. The FDG uptake of the tumor maintained a sufficiently high level for visual clinical diagnosis in most cases, except in the cases of low FDG-avid tumors or small lesions (15 mm in size).



Similar content being viewed by others
References
Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncol. 2001;3:157–64.
Wahl RL, Henry CA, Ethier SP. Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-d-glucose in rodents with mammary carcinoma. Radiology. 1992;183:643–7.
Torizuka T, Clavo AC, Wahl RL. Effect of hyperglycemia on in vitro tumor uptake of tritiated FDG, thymidine, l-methionine and l-leucine. J Nucl Med. 1997;38:382–6.
Zhao S, Kuge Y, Tsukamoto E, Mochizuki T, Kato T, Hikosaka K, et al. Effects of insulin and glucose loading on FDG uptake in experimental malignant tumours and inflammatory lesions. Eur J Nucl Med. 2001;28:730–5.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med. 1993;34:1–6.
Diederichs CG, Staib L, Glatting G, Beger HG, Reske SN. FDG-PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med. 1998;39:1030–3.
Zimny M, Bares R, Fass J, Adam G, Cremerius U, Dohmen B, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med. 1997;24:678–82.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–95.
Kitano H, Magata Y, Tanaka A, Mukai T, Kuge Y, Nagatsu K, et al. Performance assessment of O-18 water purifier. Ann Nucl Med. 2001;15:75–8.
Gorenberg M, Hallett WA, O’Doherty MJ. Does diabetes affect [(18)F]FDG standardised uptake values in lung cancer? Eur J Nucl Med Mol Imaging. 2002;29:1324–7.
Chang YC, Yen TC, Ng KK, See LC, Lai CH, Chang TC, et al. Does diabetes mellitus influence the efficacy of FDG-PET in the diagnosis of cervical cancer? Eur J Nucl Med Mol Imaging. 2005;32:647–52.
Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surgery. 2006;30:1736–41.
Higashi T, Saga T, Nakamoto Y, Ishimori T, Fujimoto K, Doi R, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET)—usefulness and limitation in “clinical reality”. Ann Nucl Med. 2003;17:261–79.
Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427–33.
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–9.
Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y, et al. [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia. 2005;7:369–79.
Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery. 2008;143:769–77.
Namageyo-Funa A, Nanavati P. The challenges of addressing primary prevention of diabetes: a response to recommendations from the chronic disease directors’ project. J Public Health Manag Pract. 2008;14:26–8.
Schiel R, Beltschikow W, Steiner T, Stein G. Diabetes, insulin, and risk of cancer. Methods Find Exp Clin Pharmacol. 2006;28:169–75.
Nishikawa T, Edelstein D, Du XL. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–90.
Guyot LL, Diaz FG, O’Regan MH, Song D, Phillis JW. The effect of topical insulin on the release of excitotoxic and other amino acids from the rat cerebral cortex during streptozotocin-induced hyperglycemic ischemia. Brain Res. 2000;872:29–36.
Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W. Increased cerebral glucose utilization and decreased glucose transporter Glut-1 during chronic hyperglycemia in rat brain. Brain Res. 2000;858:338–47.
Alpert E, Gruzman A, Totary H, Kaiser N, Reich R, Sasson S. A natural protective mechanism against hyperglycemia in vascular endothelial and smooth-muscle cells: role of glucose and 12-hydroxyeicosatetraenoic acid. Biochem J. 2002;362:413–22.
Nagamatsu S, Sawa H, Wakizaka A, Hoshino T. Expression of facilitative glucose transporter isoforms in human brain tumors. J Neurochem. 1993;61:2048–53.
Peiró C, Lafuente N, Matesanz N, Cercas E, Llergo JL, Vallejo S, et al. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br J Pharmacol. 2001;133:967–74.
Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hara, T., Higashi, T., Nakamoto, Y. et al. Significance of chronic marked hyperglycemia on FDG-PET: is it really problematic for clinical oncologic imaging?. Ann Nucl Med 23, 657–669 (2009). https://doi.org/10.1007/s12149-009-0288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-009-0288-7