Abstract
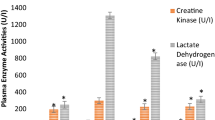
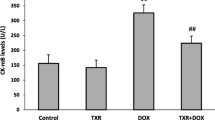
Generation of reactive oxygen species and mitochondrial dysfunction has been implicated in adriamycin induced cardiotoxicity. Mitochondrial dysfunction is characterized by the accumulation of oxidized lipids, proteins and DNA, leading to disorganization of mitochondrial structure and systolic failure. The present study was aimed to evaluate the efficacy of Centella asiatica on the mitochondrial enzymes; mitochondrial antioxidant status in adriamycin induced myocardial injury. Adriamycin (2.5 mg/kg body wt., i.p.) induced mitochondrial damage in rats was assessed in terms of decreased activities (p< 0.05) of cardiac marker enzymes (lactate dehydrogenase, creatine phosphokinase, amino transferases), TCA cycle enzymes (isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, malate dehydrogenase, respiratory marker enzymes (NADH-dehydrogenase, cytochrome-C-oxidase), mitochondrial antioxidant enzymes (GPx, GSH, SOD,CAT) and increased (p< 0.05) level of lipid peroxidation. Mitochondrial damage was confirmed by transmission electron microscopic examination. Pre-co-treatment with aqueous extract of Centella asiatica (200 mg/kg body wt, oral) effectively counteracted the alterations in mitochondrial enzymes and mitochondrial defense system. In addition, transmission electron microscopy study confirms the restoration of cellular normalcy and accredits the cytoprotective role of Centella asiatica against adriamycin induced myocardial injury. Our results demonstrated elevated oxidative stress and mitochondrial dysfunction in adriamycin treated rats. Moreover, on the basis of our findings it may be concluded that the aqueous extract of C. asiatica not only possesses antioxidant properties but it may also reduce the extent of mitochondrial damage
Similar content being viewed by others
References
Singal PK, Li T, Kumar D, Danelisen I, Iiskovic N: Adriamycin-induced heart failure: Mechanisms and modulation. Mol Cell Biochem 207: 77–85, 2000
Singal PK, Iliskovic N, Li T, Kumar D: Adriamycin cardiomyopathy: Pathophysiology and Prevention. FASEB J 11: 931–936, 1997
Singal PK, Iliskovic N: Doxorubicin induced cardiomyopathy. New Eng J Med 339: 900–905, 1998
Cassidy SC, Chan DP, Rowland DG, Allen HD: Effects of doxorubicin on diastolic function, contractile reserve, and ventricular-vascular coupling in piglets. Ped Cardiol 19: 450–457, 1998
Doroshow JH: Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res 43: 460–472, 1983
Nicolay K, De Kruijff B: Effects of adriamycin on respiratory chain activities in mitochondria from rat liver, rat heart and bovine heart. Evidence for a preferential inhibition of complex III and IV. Biochem Biophy Acta 892: 320–330, 1987
Kotamraju S, Konorev H, Joseph B, Kalyanaraman B: Doxorubicin induced apoptosis in endothelial cells and cardiomyocytes. Role of reactive oxygen and nitrogen species. J Biol Chem 275: 33585–33592, 2000
Ji LL, Mitchell EW: Effects of adriamycin on heart mitochondrial in rested and exercised rats. Biochem Pharmacol 47: 877–885, 1994
Nicolay K, Fok JJ, Voorhout W, Post JA, Kruijff B: Cytofluorescence detection of adriamycin-mitochondria interactions in isolated, perfused rat heart. Biochem Biophy Acta 887: 35–41, 1986
Cebalas-picot I, Nicole A, Clement M, Bourke JM, Signet PM: Age-related changes in antioxidant enzymes and lipid peroxidation in brains of control and transgenic mice over expressing copper—zinc superoxide dismutase. Mut Res 275: 281–293, 1992
Bast A, Haenen GR, Doelman CJ: Oxidants and antioxidants: state of the art. Am. J. Med 30: 2S–13S, 1991
Madavi DL, Salunkhe, DK: Toxicological aspects of food antioxidant. In: Madhavi, D.L., Deshpande, SS, Salunkhe, DK. (Eds.), Food Antioxidants. Marcel Dekker, New York, 1995, p. 267
Youdimx KA, Joseph JA: A possible emerging role of phyto chemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radical Biol Med 30: 583–594, 2001
Babu TD, Kuttan G, Padikkala J: Cytotoxic and antitumor properties of certain texa of umbelliferae with specific reference to Centella asiatica (L.) urban. J Ethnopharmacol 48: 3–57, 1995
Sairam K, Rao V.Ch, Goel RK: Effect of Centella asiatica Linn on physical and chemical factors induced gastric ulceration and secretion in rats. Ind J Exp Biol 39: 137–142, 2001
Suguna L, Sivakumar P, Chandrakasan G: Effects of Centella asiatica extract on dermal wound healing in rats. Ind J Exp Biol 34: 208–11, 1996
Incandela L, Belcaro G, Cesarone MR, De-Sanctis MT, Santavenere CD, Auro MG, Bucci M, Belcaro G: Total triterpenic fraction of Centella asiatica in chronic venous insufficiency and high-perfusion microangiopathy. Angiology 52 (Suppl. 2): S9–S13, 2001
Pragada RR, Veeravalli KK, Chowdary KPR, Routhu KV: Cardioprotective activity of Hydrocotyle asiatica (L) in ischemia-reperfusion induced myocardial infarction in rats. J Ethnopharmacol 93: 105–108, 2004
Jayashree G, Kurup MG, Sudarslal VS, Jacob VB: Antioxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia 74: 431–434, 2003
Veerendra Kumar MH, Gupta YK: Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol 79: 253–260, 2002
Zainol MK, Abd-Hamid A, Yusof S, Muse R: Antioxidative activity and total phenolic compounds of leaf, root, and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem 81: 575–581,2003
Hansen K, Nyman U, Smitt W, Adsersen A, Gudiksen L, Rajasekharan S, Pushpangadan P: In vitro screening of tradional medicines for antihypertensive affect based on inhibition of the angiotension converting enzymes (ACE). J Ethnopharmacol 48: 43–51, 1995
Inamdar PK, Yeole RD, Ghogare AB, de Souza NJ: Determination of biologically active constituents in Centella asiatica. J Chromatography A 42: 27–130, 1996
Cheng CL, Guo JS, Luk J, Koo MWL: The healing effect of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci 74: 2237–2249, 2004
Gnanapragasam A, Kumar Ebenezar K, Sathish V, Govidaraju P, Devaki T: Protective effect of Centella asiatica on antioxitant tissue defense system against adriamycin induced cardiomyopathy in rats. Life Sci 765: 85–597, 2004
Gupta YK, Veerendrakumar MH, Srivastava AK: Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress. Pharmacol Biochem Behav 74: 579–584, 2003
Siveski-Iliskovic N, Kaul N, Singal PK: Probucol promotes endogenous antioxidants and provides protection against adriamycin induced cardiomyopathy in rats. Circulation 89: 2829–2835, 1994
Siveski-Iliskovic N, Hill M, Chow DA, Singal PK: Probucol protects against adriamycin cardiomyopathy without interfering with its anti-tumor properties. Circulation 91: 10–15, 1995
King J: The dehydrogenase of oxido reductase lactate dehydrogenase. In: Practical clinical enzymology. (Ed.) Van D. Nostrand Co, London. 1965a. pp.83–93
Okinaka S, Kumogai H, Ebashi S, Sugita H, Mornoi H, Toyokura Y, Fujie Y: Serum creatine phosphokinase activity in progressive muscular dystrophy and neuro muscular diseases. Arch Neurol 4: 520–525,1961
Bergmeyer HV, Bernt E: Amino transferases and related enzymes. In: Methods of enzymatic analysis. (Ed.) Bergmeyer HV, Vol.2, 2nd edn, Academic Press, New York. 1974, pp. 735–763
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ: Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275,1951
Johnson D, Lardy H: Isolation of liver or kidney mitochondria. In: Methods in enzymology. (Ed.) Estabrook RW. Vol.10, Academic Press,London.1947, pp. 94–96
King J: Isocitrate dehydrogenase. In: Practical clinical enzymology. (Ed.) Van D. Nostrand Co, London, 1965b, p.363
Mehler AH, Kornberg A, Grisolia, S, Ochoa S: The enzymatic mechanism of oxidation-reductions between malate or isocitrate or pyruvate. J Biol Chem 174: 961–977, 1948
Slater EC, Bonner WD: The effect of fluoride on the succinic oxidase system. Biochem. J 52: 185–196, 1952
Reed LJ, Mukherjee RB: ́-Ketoglutarate dehydrogenase complex from Escherichia coli. In: Methods in Enzymology. (Ed.) Lowenstein JM. Vol.13, Academic Press, London, 1969, pp. 53–61
Minakami S, Ringler RL, Singer TP: Studies on the respiratory chain-linked dihydro diphospho pyridine nucleotide dehydrogenase I. Assay of the enzyme in particulate and insoluble preparations. J Biol Chem 237: 569–576, 1962
Pearl W, Cancercao J, Zweifach BW: Micro determination of cytochrome oxidase in rat tissues by the oxidation of N-phenyl-p-phenylene diamine of ascorbic acid. J Hist Cytochem 11: 102–104, 1963
Ballentine R, Burford DD: Determination of metals. In: Methods in Enzymology. (Eds.) Colowick SP and Kaplan. NO. Vol.3, Academic Press, New York, 1957. Pp, 1002–1035
Ellman GL: Tissue sulphydryl groups. Arch Biochem Biophy 82: 70–77, 1959
Paglia DE, Valentaine WN: Studies on the glutathione and glutathione characterization of erythrocyte glutathione peroxidase. J Lab Clin.Meth 70: 158–159, 1967
Habig WH, Pabst MJ, Jakoby WB: Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139, 1974
Hodgson EK, Fridovich I: The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescences and peroxidation. Biochem 14: 5399–5303, 1975
Takahara S, Hamilton BM, Nell JV, Ogura Y, Nishimura ET: Hypo-catalasemia, a new genetic carrier states. J Clin Invest 29: 610–619, 1960
Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358,1979
Ebenezar KK, Sathish V, Devaki T: Effect of arginine and lysine on mitochondrial function during isoproterenol-induced myocardial infarction in rats. Nut Res 23: 1269–1277, 2003
Oliveira PJ, Bjork AJ, Santos SM, Leino RL, Kent Froberg M, Moreno AJ, Wallace KB: Carvidilol mediated antioxdant protection against doxorubicin induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol 200(2): 159–168, 2004
Balanehru S, Nagarajan B: Intervention of adriamycin induced free radical damage. Biochem Int 28: 735–744, 1992
Stadtman ER: Protein oxidation and aging. Science 257: 1220–1224, 1992
Mimnaugh EG, Trush MA, Mohit Bhatnagar Gram FE: Enhancement of reactive oxygen dependant mitochondrial membrane lipid peroxidation by the anti cancer drug adriamyin. Biochem Pharmacol 34: 847–856, 1985
Malarkodi KP, Balachander AV, Varalakshmi P: The influence of lipoic acid on adriamycin induced nephotoxicity in rats. Mol Cell Biochem 247: 15–22, 2003
Vijayapadma V, Shyamaladevi CS: Effect of fish oil on mitochondrial respiration in isoproterenol induced myocardial infarction in rats. Ind J Exp Biol 40: 268–272, 2001
Sathish V, Vimal V, Ebenezar KK, Devaki T: Synergistic effect of nicorandil and amlodipine on mitochondrial function during isoproterenol-induced myocardial infarction in rats. J Pharm Pharmacol 54: 133–137, 2002
Solem LE, Heller LJ, Wallace KB: Dose-dependent increase in sensitivity to calcium induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J Mol Cell Cardiol 28: 1023–1032,1996
Zhou S, Heller LJ, Wallace KB: Interference with calcium dependent mitochondrial bioenergetics in cardiac myocytes isolated from doxorubicin treated rats. Toxicol App Pharmacol 175: 60–67, 2001
Iliskovic N, Hasinoff BB, Malisza KL, Li T, Danelisen I, Singal PK: Mechanisms of beneficial effects of probucol in adriamycin cardiomyopathy. Mol Cell Biochem 196: 43–49, 1999
Kyle ME, Nakal D, Saskaida I, Serroni A, Farber JL: Protein thiol depletion and the killing of cultured hepatocytes by hydrogen peroxide. Biochem Pharmacol 38: 3797–3805, 1989
Revis NW, Marusic N: Glutathione peroxidase activity and selenium concentration in the hearts of doxorubicin-treated rabbits. J Mol Cell Cardiol 10: 945–951, 1978
Yoshida M, Fuchigami M, Nagao T, Okabe H et al: Antiproliferative constituents from umbelliferae plants, active triterpenoids and rosamarinic acid from Centella asiatica. Biol Pharm Pull 28(1): 173–175, 2005
Deepa PR, Varalakshmi P: Protective effect of low molecular weight heparin on oxidative injury and cellular abnormalities in adriamycin-induced cardiac and hepatic toxicity. Chemico-Biol Int 146: 201–210, 2003
Subathra M, Shila S Anusuya Devi M, Panneerselvam C: Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp Gerontol: 1–9 (in press), 2005
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F: Flavonoids as antioxidant agents: importance of their interactions with biomembranes. Free Rad Biol Med19: 481–486, 1995
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gnanapragasam, A., Yogeeta, S., Subhashini, R. et al. Adriamycin induced myocardial failure in rats: Protective role of Centella asiatica . Mol Cell Biochem 294, 55–63 (2007). https://doi.org/10.1007/s11010-006-9245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-006-9245-0