Abstract
Purpose
Renal radiation during peptide receptor radionuclide therapy (PRRT) may result in glomerular damage, a potential reduction of glomerular filtration rate (GFR) and ultimately lead to renal failure. While reported PRRT nephrotoxicity is limited to data derived from serum creatinine—allowing only approximate estimates of GFR—the aim of this study is to accurately determine PRRT-induced long-term changes of renal function and associated risk factors according to state-of-the-art GFR measurement.
Methods
Nephrotoxicity was analysed using 99mTc-diethylenetriaminepentaacetic acid (DTPA) clearance data of 74 consecutive patients with gastroenteropancreatic neuroendocrine tumours (GEP NET) undergoing PRRT with 177Lu-octreotate. The mean follow-up period was 21 months (range 12–50) with a median of five GFR measurements per patient. The change of GFR was analysed by linear curve fit. Potential risk factors including diabetes mellitus, arterial hypertension, previous chemotherapy, renal impairment at baseline and cumulative administered activity were analysed regarding potential impact on renal function loss. In addition, Common Terminology Criteria for Adverse Events (CTCAE) v3.0 were used to compare nephrotoxicity determined by 99mTc-DTPA clearance versus serum creatinine.
Results
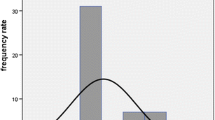
The alteration in GFR differed widely among the patients (mean −2.1 ± 13.1 ml/min/m2 per year, relative yearly reduction −1.8 ± 18.9 %). Fifteen patients (21 %) experienced a mild (2–10 ml/min/m2 per year) and 16 patients (22 %) a significant (>10 ml/min/m2 per year) decline of GFR following PRRT. However, 11 patients (15 %) showed an increase of >10 ml/min/m2 per year. Relevant nephrotoxicity according to CTCAE (grade ≥3) was observed in one patient (1.3 %) with arterial hypertension and history of chemotherapy. Nephrotoxicity according to serum creatinine was discordant to that defined by GFR in 15 % of the assessments and led to underestimation in 12 % of patients. None of the investigated factors including cumulative administered activity contributed to the decline of renal function.
Conclusion
Serious nephrotoxicity after PRRT with 177Lu-octreotate is rare (1.3 %). However, slight renal impairment (GFR loss >2 ml/min/m2 per year) can frequently (43 %) be detected by 99mTc-DTPA clearance assessments. Cumulative administered activity of 177Lu-octreotate is not a major determinant of renal impairment in our study.


Similar content being viewed by others
References
Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 2010;40(2):78–88. doi:10.1053/j.semnuclmed.2009.10.004.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26(13):2124–30. doi:10.1200/JCO.2007.15.2553.
Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40(1):173–85, ix. doi:10.1016/j.ecl.2010.12.003.
Buscombe JR, Caplin ME, Hilson AJ. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med 2003;44(1):1–6.
Delpassand ES, Samarghandi A, Mourtada JS, Zamanian S, Espenan GD, Sharif R, et al. Long-term survival, toxicity profile, and role of F-18 FDG PET/CT scan in patients with progressive neuroendocrine tumors following peptide receptor radionuclide therapy with high activity In-111 pentetreotide. Theranostics 2012;2(5):472–80. doi:10.7150/thno.3739.
Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001;12(7):941–5.
Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med 2002;43(5):610–6.
Ezziddin S, Sabet A, Heinemann F, Yong-Hing CJ, Ahmadzadehfar H, Guhlke S, et al. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J Nucl Med 2011;52(8):1197–203. doi:10.2967/jnumed.111.090373.
Ezziddin S, Opitz M, Attassi M, Biermann K, Sabet A, Guhlke S, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2011;38(3):459–66. doi:10.1007/s00259-010-1610-2.
Bernard BF, Krenning EP, Breeman WA, Rolleman EJ, Bakker WH, Visser TJ, et al. D-lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med 1997;38(12):1929–33.
de Jong M, Krenning E. New advances in peptide receptor radionuclide therapy. J Nucl Med 2002;43(5):617–20.
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med 2001;28(4):426–34.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26(11):1439–47.
Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with 90Y-DOTATOC. Eur J Nucl Med 2001;28(10):1552–4. doi:10.1007/s002590100599.
Cwikla JB, Sankowski A, Seklecka N, Buscombe JR, Nasierowska-Guttmejer A, Jeziorski KG, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol 2010;21(4):787–94. doi:10.1093/annonc/mdp372.
Moll S, Nickeleit V, Mueller-Brand J, Brunner FP, Maecke HR, Mihatsch MJ. A new cause of renal thrombotic microangiopathy: yttrium 90-DOTATOC internal radiotherapy. Am J Kidney Dis 2001;37(4):847–51.
Stoffel MP, Pollok M, Fries J, Baldamus CA. Radiation nephropathy after radiotherapy in metastatic medullary thyroid carcinoma. Nephrol Dial Transplant 2001;16(5):1082–3.
Behr TM, Behe MP. Cholecystokinin-B/gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Semin Nucl Med 2002;32(2):97–109. doi:10.1053/snuc.2002.31028.
Forrer F, Rolleman E, Bijster M, Melis M, Bernard B, Krenning EP, et al. From outside to inside? Dose-dependent renal tubular damage after high-dose peptide receptor radionuclide therapy in rats measured with in vivo (99m)Tc-DMSA-SPECT and molecular imaging. Cancer Biother Radiopharm 2007;22(1):40–9. doi:10.1089/cbr.2006.353.
Rolleman EJ, Krenning EP, Bernard BF, de Visser M, Bijster M, Visser TJ, et al. Long-term toxicity of [(177)Lu-DOTA (0), Tyr (3)]octreotate in rats. Eur J Nucl Med Mol Imaging 2007;34(2):219–27. doi:10.1007/s00259-006-0232-1.
Pöge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP. MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 2005;5(6):1306–11. doi:10.1111/j.1600-6143.2005.00861.x.
Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, et al. [177Lu-DOTAOTyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med 2001;28(9):1319–25.
Forrer F, Uusijärvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med 2005;46(8):1310–6.
Kwekkeboom DJ, Bakker WH, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0), Tyr3]octreotate. Eur J Nucl Med Mol Imaging 2003;30(3):417–22. doi:10.1007/s00259-002-1050-8.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 2008;35(10):1847–56. doi:10.1007/s00259-008-0778-1.
Flynn AA, Pedley RB, Green AJ, Dearling JL, El-Emir E, Boxer GM, et al. The nonuniformity of antibody distribution in the kidney and its influence on dosimetry. Radiat Res 2003;159(2):182–9.
Breeman WA, De Jong M, Visser TJ, Erion JL, Krenning EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging 2003;30(6):917–20. doi:10.1007/s00259-003-1142-0.
Breeman WA, van der Wansem K, Bernard BF, van Gameren A, Erion JL, Visser TJ, et al. The addition of DTPA to [177Lu-DOTA0, Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging 2003;30(2):312–5. doi:10.1007/s00259-002-1054-4.
Russell CD, Bischoff PG, Kontzen FN, Rowell KL, Yester MV, Lloyd LK, et al. Measurement of glomerular filtration rate: single injection plasma clearance method without urine collection. J Nucl Med 1985;26(11):1243–7.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with (177)Lu-DOTATATE: the IEO phase I–II study. Eur J Nucl Med Mol Imaging 2011;38(12):2125–35. doi:10.1007/s00259-011-1902-1.
Gupta SK, Singla S, Bal C. Renal and hematological toxicity in patients of neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-DOTATATE. Cancer Biother Radiopharm 2012;27(9):593–9. doi:10.1089/cbr.2012.1195.
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0), Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med 2005;46 Suppl 1:83S–91S.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23(12):2754–62. doi:10.1200/JCO.2005.08.066.
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29(17):2416–23. doi:10.1200/JCO.2010.33.7873.
Frilling A, Weber F, Saner F, Bockisch A, Hofmann M, Mueller-Brand J, et al. Treatment with (90)Y- and (177)Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery 2006;140(6):968–76. doi:10.1016/j.surg.2006.07.030. discussion 76–77.
Acknowledgments
The authors are grateful to Professor Eric Krenning, Professor Dik Kwekkeboom and Professor Wouter A. P. Breeman (Erasmus Medical Center, Rotterdam, Netherlands) for sharing their experience in the receptor targeting field and making somatostatin receptor-mediated treatment in our institution possible. Also, we thank Professor Richard P. Baum (Department of Nuclear Medicine and PET Center, Zentralklinik Bad Berka, Germany) for his critical and constructive input in this field. The authors also are thankful to the personnel of the Nuclear Medicine Department and especially the nursing staff of the therapy ward.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabet, A., Ezziddin, K., Pape, UF. et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. Eur J Nucl Med Mol Imaging 41, 505–510 (2014). https://doi.org/10.1007/s00259-013-2601-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2601-x