Abstract
Purpose
Reconstruction of DaTSCAN brain studies using OS-EM iterative reconstruction offers better image quality and more accurate quantification than filtered back-projection. However, reconstruction must proceed for a sufficient number of iterations to achieve stable and accurate data. This study assessed the impact of the number of iterations on the image quantification, comparing the results of the iterative reconstruction with filtered back-projection data.
Methods
A striatal phantom filled with 123I using striatal to background ratios between 2:1 and 10:1 was imaged on five different gamma camera systems. Data from each system were reconstructed using OS-EM (which included depth-independent resolution recovery) with various combinations of iterations and subsets to achieve up to 200 EM-equivalent iterations and with filtered back-projection. Using volume of interest analysis, the relationships between image reconstruction strategy and quantification of striatal uptake were assessed.
Results
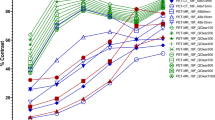
For phantom filling ratios of 5:1 or less, significant convergence of measured ratios occurred close to 100 EM-equivalent iterations, whereas for higher filling ratios, measured uptake ratios did not display a convergence pattern. Assessment of the count concentrations used to derive the measured uptake ratio showed that nonconvergence of low background count concentrations caused peaking in higher measured uptake ratios. Compared to filtered back-projection, OS-EM displayed larger uptake ratios because of the resolution recovery applied in the iterative algorithm.
Conclusion
The number of EM-equivalent iterations used in OS-EM reconstruction influences the quantification of DaTSCAN studies because of incomplete convergence and possible bias in areas of low activity due to the nonnegativity constraint in OS-EM reconstruction. Nevertheless, OS-EM using 100 EM-equivalent iterations provides the best linear discriminatory measure to quantify the uptake in DaTSCAN studies.












Similar content being viewed by others
References
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62(2):133–40.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6(4):305–13.
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 2000;15(3):503–10.
Schneider SA, Edwards MJ, Mir P, Cordivari C, Hooker J, Dickson J, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 2007;22(15):2210–5.
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 2001;28(3):266–72.
Chouker M, Tatsch K, Linke R, Pogarell O, Hahn K, Schwarz J. Striatal dopamine transporter binding in early to moderately advanced Parkinson’s disease: monitoring of disease progression over 2 years. Nucl Med Commun 2001;22(6):721–5.
Koch W, Hamann C, Welsch J, Pöpperl G, Radau PE, Tatsch K. Is iterative reconstruction an alternative to filtered backprojection in routine processing of dopamine transporter SPECT studies? J Nucl Med 2005;46(11):1804–11.
Crespo C, Gallego J, Cot A, Falcón C, Bullich S, Pareto D, et al. Quantification of dopaminergic neurotransmission SPECT studies with (123)I-labelled radioligands. A comparison between different imaging systems and data acquisition protocols using Monte Carlo simulation. Eur J Nucl Med Mol Imaging 2008;35(7):1334–42.
Hutton BF, Nuyts J, Zaidi H. Iterative reconstruction methods. In: Zaidi H, editor. Quantitative analysis in nuclear medicine imaging. New York: Springer; 2006. p. 107–40.
Wallis J, Miller T, Dai G. Comparison of the convergence properties of the It-W and OS-EM algorithms in SPECT. IEEE Trans Nucl Sci 1998;45(3):1317–23.
Hudson H, Larkin R. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994;13(4):601–9.
Koch W, Radau PE, Hamann C, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 2005;46(7):1109–18.
Buvat I. A non-parametric bootstrap approach for analysing the statistical properties of SPECT and PET images. Phys Med Biol 2002;47(10):1761–75.
Bélanger M, Mann JJ, Parsey RV. OS-EM and FBP reconstructions at low count rates: effect on 3D PET studies of [11C] WAY-100635. Neuroimage 2004;21(1):244–50.
Reilhac A, Tomeï S, Buvat I, Michel C, Keheren F, Costes N. Simulation-based evaluation of OSEM iterative reconstruction methods in dynamic brain PET studies. Neuroimage 2008;39(1):359–68.
Byrne C. Iterative algorithms for deblurring and deconvolution with constraints. Inverse Problems 1998;14(6):1455–67.
Erlandsson K, Visvikis D, Waddington W, Cullum I, Jarritt P, Polowsky L. Low-statistics reconstruction with AB-EMML. Nuclear Science Symposium Conference Record, 2000 IEEE. 2000. p. 15/249–15/253 vol.2.
Pareto D, Cot A, Pavía J, Falcón C, Juvells I, Lomeña F, et al. Iterative reconstruction with correction of the spatially variant fan-beam collimator response in neurotransmission SPET imaging. Eur J Nucl Med Mol Imaging 2003;30(10):1322–9.
Du Y, Tsui BMW, Frey EC. Model-based compensation for quantitative 123I brain SPECT imaging. Phys Med Biol 2006;51(5):1269–82.
Cot A, Falcón C, Crespo C, Sempau J, Pareto D, Bullich S, et al. Absolute quantification in dopaminergic neurotransmission SPECT using a Monte Carlo-based scatter correction and fully 3-dimensional reconstruction. J Nucl Med 2005;46(9):1497–504.
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with (123)I SPECT. J Nucl Med 2003;44(7):1184–93.
Jarritt PH, Whalley DR, Skrypniuk JV, Houston AS, Fleming JS, Cosgriff PS. UK audit of single photon emission computed tomography reconstruction software using software generated phantoms. Nucl Med Commun 2002;23(5):483–91.
Acknowledgments
The authors would like to thank Michel Koole from the Nuclear Medicine Department, UZ Gasthuisberg, Leuven, and Gerda Thomsen from the Neurobiology Research Unit, Rigshospitalet, Copenhagen, for providing phantom data for this study.
This investigation was part of a project launched by EANM Research Ltd (EARL)/European Network of Excellence for Brain Imaging whose aim is to create a European Normal Control Database of DaTSCAN (ENC-DAT). More details can be found at http://www.eanm.org/earl/pilot.php?navId=497. EARL/European Network of Excellence for Brain Imaging would like to thank GE Healthcare and the German Parkinson Association for their financial contribution to this study. This work was undertaken at UCLH/UCL where a proportion of the funding was received from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dickson, J.C., Tossici-Bolt, L., Sera, T. et al. The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging 37, 23–35 (2010). https://doi.org/10.1007/s00259-009-1212-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1212-z