Abstract
Purpose
The aim of this study was to evaluate the accuracy of different single-photon emission computed tomography (SPECT) reconstruction techniques in measuring striatal N-ω-fluoropropyl-2β-carbomethoxy-3β-4-[123I]iodophenyl-nortropane (123I-FP-CIT) binding in de novo Parkinson’s disease (PD) patients, in order to find a correlation with clinical scales of disease severity in the initial phases of disease.
Methods
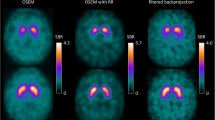
Thirty-six de novo PD patients underwent 123I-FP-CIT SPECT and MRI scan. SPECT data were reconstructed with filtered back projection (FBP), with an iterative algorithm (ordered subset expected maximization, OSEM) and with a method previously developed in our institution, called least-squares (LS) method. The ratio of specific to non-specific striatal 123I-FP-CIT binding (binding potential, BP) was used as the outcome measure with all the reconstruction methods (BPFBP, BPOSEM, BPLS).
Results
The range of values of striatal BPLS was significantly greater than BPFBP and BPOSEM. For all striatal regions, estimates of BPFBP correlated well with BPOSEM (r = 0.84) and with BPLS (r = 0.64); BPOSEM correlated significantly with BPLS (r = 0.76). A good correlation was found between putaminal BPLS and Hoen and Yahr, Unified PD Rating Scale (UPDRS) and lateralized UPDRS motor scores (r = −0.46, r = −0.42, r = −0.39, respectively). Neither putaminal BPFBP nor putaminal BPOSEM correlated with any of these motor scores.
Conclusions
In de novo PD patients, 123I-FP-CIT BP values derived from FBP and OSEM reconstruction techniques do not permit to differentiate PD severity. The LS method instead finds a correlation between striatal BP and disease severity scores. The results of this study support the use of 123I-FP-CIT BP values estimated with the LS method as a biomarker of PD severity.






Similar content being viewed by others
References
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 1991;114:2283–301.
Kaufman MJ, Madras BK. Severe depletion of cocaine recognition sites associated with the dopamine transporter in Parkinson’s disease striatum. Synapse 1991;9:43–9.
Seibyl JP. Imaging studies in movement disorders. Semin Nucl Med 2003;33:105–13.
Seibyl JP, Marek K, Sheff K, Zoghbi S, Baldwin RM, Charney DS, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurements of dopamine transporters in healthy subjects and Parkinson’s patients. J Nucl Med 1998;39:1500–8.
Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord 2003;18:1415–23.
Benamer HTS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 2000;15:503–10.
Plotkin M, Amthauer H, Klaffke S, Kuhn A, Ludemann L, Arnold G, et al. Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm 2005;112(5):677–92.
Booij J, Habraken JB, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med 1998;39(11):1879–84.
Booij J, Tissingh G, Winogrodzka A, Boer GJ, Stoof JC, Wolters EC, et al. Practical benefit of [123I]FP-CIT SPET in the demonstration of the dopaminergic deficit in Parkinson’s disease. Eur J Nucl Med 1997;24:68–71.
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen A, Van Royen E, et al. Iodine-123-N-ω-fluoropropyl-2-β-carbomethoxy-3β-(4-iodophenyl)tropane SPECT in healthy controls and early stage, drug-naive Parkinson’s disease. J Nucl Med 1998;39:1143–8.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27(9):1533–9.
Booij J, Speelman JD, Horstink MWIM, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 2001;28:266–72.
Isaias IU, Benti R, Cilia R, Canesi M, Marotta G, Gerundini P, et al. [123I]FP-CIT striatal binding in early Parkinson’s disease patients with tremor vs. akinetic-rigid onset. NeuroReport 2007;18:1499–502.
Koch W, Randau PE, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 2005;46(7):1109–18.
Michell AW, Lewis SJG, Foltynie T, Barker RA. Biomarkers and Parkinson’s disease. Brain 2004;127:1693–705.
Spiegel J, Hellwig G, Samnick S, Jost W, Mollers MO, Fassbender K, et al. Striatal FP-CIT uptake differs in subtypes of early Parkinson’s disease. J Neural Transm 2007;114(3):331–5.
Eshuis SA, Maguire RP, Leenders KL, Jonkman S, Jager PL. Comparison of FP-CIT SPECT with F-DOPA PET in patients with de novo and advanced Parkinson’s disease. Eur J Nucl Med Mol Imaging 2006;33:200–9.
Benamer HTS, Patterson J, Wyper DJ, Hadley DM, Macphee GJA, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 2000;15:692–8.
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with 123I SPECT. J Nucl Med 2003;44(7):1184–93.
Formiconi AR, Passeri A, Calvini P. Theoretical determination of the collimator geometrical transfer function for the reconstruction of SPECT data. IEEE Trans Nucl Sci 1999;46(4):1075–80.
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39(5):904–11.
Formiconi AR. Least squares algorithm for region of interest evaluation in emission tomography. IEEE Trans Med Imag 1993;2(1):90–100.
Vanzi E, De Cristofaro MT, Ramat S, Sotgia B, Mascalchi M, Formiconi AR. A direct ROI quantification method for inherent PVE correction: accuracy assessment in striatal SPECT measurements. Eur J Nucl Med Mol Imaging 2007;34(9):1480–9.
Hughes A, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinico-pathological study. Neurology 1992;42:1142–6.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42.
Fahn S, Elton RL, and members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent development in Parkinson’s disease. Florham Park, HJ: Macmillan Healthcare Information; 1987. p. 153–64.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Transm Med Imaging 1994;13(4):601.
Chang L. A method for attenuation correction in computed tomography. IEEE Trans Nucl Sci 1987;25:638–64.
National Institutes of Health. Medical image processing, analysis and visualization. www.mipav.cit.nih.gov (as achieved in January 24th, 2008)
McAuliffe MJ, Lalonde D, McGarry D, Gandler W, Csaky K, Trus BL. Medical image processing, analysis and visualization in clinical research. In: CBMS’01: 2001 Proceedings of the IEEE Computer-based Medical Systems, Bethesda, MD, p. 381.
Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, et al. Graphical, kinetic and equilibrium analyses of in vivo [123I]β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994;14:982–94.
Pirker W. Correlation of dopamine transporter imaging and parkinsonian motor handicap: how close is it? Mov Disord 2003;18(Suppl 7):S43–51.
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, et al. Decreased single photon emission computed tomographic [123I]β-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 1995;38:589–98.
Brucke T, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wober C, et al. Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I]β-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm 1997;50(Suppl):9–24.
Asenbaum S, Brucke T, Pirker W, Podreka I, Angelberger P, Wenger S, et al. Imaging of dopamine transporters with iodine-123-β-CIT and SPECT in Parkinson’s disease. J Nucl Med 1997;38:1–6.
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berti, V., Pupi, A., Ramat, S. et al. Clinical correlation of the binding potential with 123I-FP-CIT in de novo idiopathic Parkinson’s disease patients. Eur J Nucl Med Mol Imaging 35, 2220–2226 (2008). https://doi.org/10.1007/s00259-008-0872-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0872-4