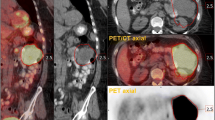
Abstract
Purpose
Functional imaging of cancer adds important information to the conventional measurements in monitoring response. Serial 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET), which indicates changes in glucose metabolism in tumours, shows great promise for this. However, there is a need for a method to quantitate alterations in uptake of FDG, which accounts for changes in tumour volume and intensity of FDG uptake. Selection of regions or volumes [ROI or volumes of interest (VOI)] by hand drawing, or simple thresholding, suffers from operator-dependent drawbacks.
Materials and methods
We present a simple, robust VOI growing method for this application. The method requires a single seed point within the visualised tumour and another in relevant normal tissue. The drawn tumour VOI is insensitive to the operator inconsistency and is, thus, a suitable basis for comparative measurements. The method is validated using a software phantom. We demonstrate the use of the method in the assessment of tumour response in 31 patients receiving chemotherapy for various carcinomas.
Results
Valid assessment of tumour response could be made 2–4 weeks after starting chemotherapy, giving information for clinical decision making which would otherwise have taken 9–12 weeks. Survival was predicted from FDG-PET 2–4 weeks after starting chemotherapy (p = 0.04) and after 9–12 weeks FDG-PET gave a better prediction of survival (p = 0.002) than CT or MRI (p = 0.015).
Conclusions
FDG-PET using this method of analysis has potential as a routine tool for optimising use of chemotherapy and improving its cost effectiveness. It also has potential for increasing the accuracy of response assessment in clinical trials of novel therapies.









Similar content being viewed by others
References
Fischman AJ. Positron emission tomography in the clinical evaluation of metastatic cancer. J Clin Oncol 1996;14:691–6.
Bombardieri E. The added value of metabolic imaging with FDG-PET in oesophageal cancer: prognostic role and prediction of response to treatment. Eur J Nucl Med Mol Imaging 2006;33:753–8.
Couper GW, McAteer D, Wallis F, Norton M, Welsh A, Nicolson M, et al. The detection of response to chemotherapy using positron emission tomography in patients with oesophageal and gastric cancer. Br J Surg 1998;85:31.
Findlay M, Young H, Cunningham D, Iveson A, Cronin B, Hickish T, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 1996;14:700–8.
Kostakoglu L, Goldsmith SJ. F-18-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med 2003;44:224–39.
Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, et al. CT and PET in stomach cancer: Preoperative staging and monitoring of response to therapy. Radiographics 2006;26:U143–98.
Mikhaeel NG. Use of FDG-PET to monitor response to chemotherapy and radiotherapy in patients with lymphomas. Eur J Nucl Med Mol Imaging 2006;33:S22–6.
Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, et al. Evaluation of liver-tumors using fluorine-18-fluorodeoxyglucose pet—characterization of tumor and assessment of effect of treatment. J Nucl Med 1992;33:333–9.
Simo M, Lomena F, Setoain J, Perez G, Castellucci P, Costansa JM, et al. FDG-PET improves the management of patients with suspected recurrence of colorectal cancer. Nucl Med Common 2002;23:975–82.
Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast-cancer chemohormonotherapy using positron emission tomography—initial evaluation. J Clin Oncol 1993;11:2101–11.
Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging 2006;33:S27–S37.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG—body-surface area correction is preferable to body-weight correction. J Nucl Med 1994;35:164–7.
Kim CK, Gupta NC. Dependency of standardized uptake values of fluorine-18 fluorodeoxyglucose on body size: Comparison of body surface area correction and lean body mass correction. Nucl Med Common 1996;17:890–4.
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–48.
Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast-carcinoma—initial clinical-evaluation with Pet with the radiolabeled glucose analog 2-[F-18]-Fluoro-2-Deoxy-d-Glucose. Radiology 1991;179:765–70.
Keyes JW. SUV—standard uptake or silly useless value. J Nucl Med 1995;36:1836–9.
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med 1999;40:1771–7.
Westerterp M, Pruim J, Oyen W, Hoekstra O, Paans A, Visser E, et al. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging 2007;34:392–404.
Mortelmans L. A new thresholding method for volume determination by SPECT. Eur J Nucl Med 1986;12:284–90.
Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med 1-5-2006;47:885–95.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519–27.
Fleming JS, Bole L, Stratford JS, Kemp PM. THe specific size index for quantifying radiopharmaceutical uptake. Phys Med Biol 2004;49:N227–N234.
Adams R, Bischof L. Seeded region growing. IEEE Trans Pattern Anal Mach Intell 1994;16:641–7.
Green AJ, Begent RHJ. Generation of voxel phantoms to investigate the effect of statistical noise on image analysis programs. Eur J Nucl Med Mol Imaging 2006; Supplement 2:S319.
Green AJ, Adamson KL, Begent RH. Validation of a gamma camera PET system for serial 18F-FDG imaging for the measurement of response to anti-cancer therapies. J Nucl Med 1999;40:1246.
Therasse P. New guidelines to evaluate the response to treatment in solid Tumors. J Natl Cancer Inst 2000;92:205–16.
SPSS. SPSS for Windows. 19-9-2001. SPSS Inc.
Julyan P. Basic Theory of GCPET. In: Hillel P (ed) Basics of gamma camera positron emission tomography. Institute of Physics and Engineering in Medicine; 2004, pp. 1–15.
Francis RJ, Byrne MJ, van der Schaaf AA, Boucek JA, Nowak AK, Phillips M, et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semi-automated 3D volume-based analysis of serial FDG PET scans. J Nucl Med 2007;48:1449–1458.
Acknowledgment
This work is supported by Cancer Research UK.
Conflict of interest statement
All work described here was carried out under UK law. All necessary ethical and regulatory approval was obtained.
We certify that no conflict of interest exists in relation to this paper.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
18F-FDG Imaging Standard Operating Procedure
-
Patient asked to fast for 5 h before scan.
-
Height, weight and blood glucose recorded.
-
150 MBq 18 F-FDG administered i.v.
-
Patient asked to rest for 90 min.
-
Two or three rotation PET scan acquired using ACAC MCD system:
-
32 azimuths per rotation;
-
30 s per azimuth:
-
Time per projection automatically adjusted for physical decay;
-
-
Attenuation correction map acquired with projections.
-
(Where patients were not able to lie still for the required time single rotation acquisitions were used.)
-
-
OS-EM reconstruction with attenuation correction:
-
Rotations “knitted” to make single torso image;
-
Knitted images corrected for variations in:
-
Injected activity;
-
Timing of injection and scan.
-
-
-
Images saved for analysis.
Rights and permissions
About this article
Cite this article
Green, A.J., Francis, R.J., Baig, S. et al. Semiautomatic volume of interest drawing for 18F-FDG image analysis—method and preliminary results. Eur J Nucl Med Mol Imaging 35, 393–406 (2008). https://doi.org/10.1007/s00259-007-0602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0602-3