Abstract
Purpose
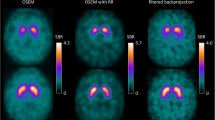
In single-photon emission computed tomography (SPECT) of the dopaminergic system, measurements of striatal uptake are useful for diagnosis and patient follow-up but are strongly biased by the partial volume effect (PVE). We studied whether PVE correction might improve patient classification based on binding potential (BP) measurements.
Methods
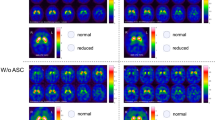
Patients with a probable diagnosis of dementia with Lewy bodies (DLB, 10 patients) or Alzheimer’s disease (AD, 13 patients) were studied by 123I-FP-CIT SPECT. SPECT images were reconstructed with and without PVE correction. Each patient SPECT scan was also simulated to obtain SPECT data whose characteristics were fully known. In addition, 17 SPECT scans were simulated with striatal uptake values mimicking pre-symptomatic cases of DLB.
Results
Without PVE correction, mean putamen BP values were 2.9±0.4 and 0.9±0.2 for AD and DLB patients respectively, while with PVE correction, they were 8.6±1.5 and 1.9±0.5 respectively. All patients were properly identified as having AD or DLB when considering mean putamen BP measured on their real or simulated SPECT scan, with and without PVE correction. All 30 simulations mimicking pre-symptomatic DLB and AD patients were accurately classified with PVE correction, but without PVE correction 15 mean putamen BP values were in a range where AD and DLB could not be distinguished.
Conclusion
We conclude that putamen BP values measured without PVE correction can be used to differentiate probable DLB and AD due to the already severe reduction in dopamine transporter levels. PVE correction appeared useful for accurate differential diagnosis between AD and pre-symptomatic DLB.





Similar content being viewed by others
References
Marek K, Seibyl J, Zoghbi S. [123I] beta-CIT SPECT imaging demonstrate bilateral loss of dopamine transporters in hemi-Parkinson’s diseases. Neurology 1996;46:231–237
Booij J, Tissingh G, Boer G, Speelman J, Stoof J, Janssen A, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62:133–140
Benamer T, Patterson J, Grosset D, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disorder 2000;15:503–510
Booij J, Tissingh G, Winogrodzka A, van Royen EA. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur J Nucl Med 1999;26:171–182
Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with 123I-FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med 2001;28:266–272
Tatsch K, Asenbaum S, Bartenstein P, Catafau AM, Halldin C, Pillowsky LS et al. European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using 123I-labelled dopamine D2 receptor ligands. Eur J Nucl Med Mol Imaging 2002;29:BP23–BP29
Tatsch K. Imaging of the dopaminergic system in parkinsonism with SPET. Nucl Med Commun 2001;22:819–827
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–1124
McKeith LG, Ballard CG, Perry RH, Ince PG, O’Brien JT, Neill D et al. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000;54:1050–1058
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol 2004;61:919–925
Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry 2002;73:134–140
Walker Z, Costa DC, Walker RW, Lee L, Livingston G, Jaros E et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology 2004;62:1568–1572
Costa DC, Walker Z, Walker RW, Fontes FR. Dementia with Lewy bodies versus Alzheimer’s disease: role of dopamine transporter imaging. Mov Disorder 2003;18:S34–S38
Tatsch K, Asenbaum S, Bartenstein P, Catafau AM, Halldin C, Pillowsky LS et al. European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using 123I-labelled dopamine D2 transporter ligands. Eur J Nucl Med Mol Imaging 2002;29:BP30–BP35
Chouker M, Tatsch K, Linke R, Pogarell O, Hahn K, Schwarz J. Striatal dopamine transporter binding in early to moderate advanced Parkinson’s disease: monitoring of disease progression over 2 years. Nucl Med Commun 2001;22:721–725
Marek K, Seibyl J, Shoulson I. Dopamine transporter brain imaging to assess the effects of Pramipexole vs levodopa on Parkinson disease progression. JAMA 2002;287:1653–1661
Hashimoto J, Sasaki T, Ogawa K, Kubo A, Motomura N, Ichihara T et al. Effects of scatter and attenuation correction on quantitative analysis of beta-CIT brain SPET. Nucl Med Commun 1999;20:159–165
Almeida P, Ribeiro MJ, Bottlaender M, Loc’h C, Langer O, Strul D et al. Absolute quantitation of iodine-123 epidepride kinetics using single-photon emission tomography: comparison with carbon-11 epidepride and positron emission tomography. Eur J Nucl Med 1999;26:1580–1588
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with 123I SPECT. J Nucl Med 2003;44:1184–1193
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 1979;3:299–308
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415–455
Prunier C, Bezard E, Montharu J, Mantzarides M, Besnard J-C, Baulieu J-L, et al. Presymptomatic diagnosis of experimental Parkinsonism with 123I-PE2I SPECT. Neuroimage 2003;19:810–816
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen E et al. Iodine-123-N-omega-fluoropropyl-2beta-carbomethoxy-3beta-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug naive Parkinson’s disease. J Nucl Med 1998;39:1143–1148
Harissson RL, Haynor DR, Gillispie SB, Vannoy SD, Kaplan MS, Lewellen TK. A public-domain simulation system for emission tomography: photon tracking throught heterogeneous attenuation using importance sampling. J Nucl Med 1993;34:60P
Ogawa K, Harata Y, Ichihara T, Kubo A, Hashimoto N. A practical method for position-dependent Compton-scattered correction in single photon emission. IEEE Trans Med Imaging 1991;10:408–412
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994;13:601–609
Tung c-H, Gullberg GT, Zeng GL, Christian PE, Datz FL, Morgan HT. Non-uniform attenuation correction using simultaneous transmission and emission converging tomography. IEEE Trans Nucl Sci 1992;39:1134–1143
Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997;16:187–198
Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39:904–911
Barber R, McKeith I, Ballard C, O’Brien J. Volumetric MRI study of the caudate nucleus in patients with dementia with Lewy bodies, Alzheimer’s disease, and vascular dementia. J Neurol Neurosurg Psychiatry 2002;72:406–407
Almeida OP, Burton EJ, McKeith I, Gholkar A, Burn D, O’Brien J. MRI study of caudate nucleus volume in Parkinson’s disease with and without dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord 2003;16:57–63
Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard C et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage 2002;17:618–630
Cousins DA, Burton EJ, Burn D, Gholkar A, McKeith I, O’Brien J. Atrophy of the putamen in dementia with Lewy bodies but not Alzheimer’s disease: an MRI study. Neurology 2003;61:1191–1195
Donnemiller E, Heilmann J, Wenning GK, Berger W, Decristoforo C, Moncayo R et al. Brain perfusion scintigraphy with 99mTc-HMPAO or 99mTc-ECD and 123I-beta-CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med 1997;24:320–325
Acknowledgment
This work was supported by the GDR CNRS – Inserm Stic-Santé (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soret, M., Koulibaly, P.M., Darcourt, J. et al. Partial volume effect correction in SPECT for striatal uptake measurements in patients with neurodegenerative diseases: impact upon patient classification. Eur J Nucl Med Mol Imaging 33, 1062–1072 (2006). https://doi.org/10.1007/s00259-005-0003-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0003-4