Abstract
Abstract
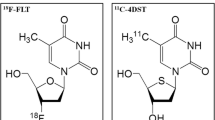
The aim of this study was to evaluate the feasibility of using [18F] 3′-deoxy-3′-fluorothymidine (FLT) positron emission tomography (PET) for the diagnosis and grading of brain tumors.
Methods
The patient population comprised 26 patients (15 males, 11 females) with brain tumors (n=18) or nontumorous lesions (n=8). 2-[18F]fluoro-2-deoxy-d-glucose (FDG) and FLT PET images were obtained using a dedicated PET scanner 1 h after the injection of 370 MBq of FDG or FLT. Uptake of FDG and FLT by the lesions was visually and semiquantitatively assessed in comparison with normal brain tissue.
Results
Of 26 brain lesions, four showed increased FDG uptake compared with normal gray matter (grade 5). These four lesions showed intensely increased FLT uptake and were all high-grade tumors. Twenty-two lesions with similar (grade 4) or decreased (grades 1–3) FDG uptake compared with normal gray matter showed variable pathology. Among the 18 brain tumors, FLT PET showed increased uptake in all 12 high-grade tumors but FDG uptake was variable. In 22 brain lesions with similar or decreased uptake compared with normal gray matter on FDG PET, the sensitivity and specificity of FLT PET for the diagnosis of brain tumor were 79% (11/14) and 63% (5/8), respectively. The uptake ratios of 14 brain tumors on FLT PET were significantly higher than the lesion to gray matter ratios (p=0.012) and lesion to white matter ratios (p=0.036) of FDG uptake and differed significantly between high (5.1±2.6) and low (2.1±1.1) grade tumors (p=0.029). In nine gliomas, FLT uptake was significantly correlated with the Ki-67 proliferation index (rho=0.817, p=0.007).
Conclusion
These findings indicate that FLT PET is useful for evaluating tumor grade and cellular proliferation in brain tumors. It displayed high sensitivity and good contrast in evaluating brain lesions that showed similar or decreased uptake compared with normal gray matter on FDG PET. FLT PET, however, did not appear to be sufficiently useful for differentiating tumors from nontumorous lesions.





Similar content being viewed by others
References
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4(11):1334–6.
Wagner M, Seitz U, Buck A, Neumaier B, Schultheiss S, Bangerter M, et al. 3′-[18F]fluoro-3′-deoxythymidine([18F]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-cell lymphoma model and in the human disease. Cancer Res 2003;63:2681–7.
Eary JF, Mankoff DA, Spence AM, Berger MS, Olshen A, Link JM, et al. 2-[C-11]thymidine imaging of malignant brain tumors. Cancer Res 1999;59:615–21.
Schifter T, Hoffman JM, Hanson MW, Boyko OB, Beam C, Paine S, et al. Serial FDG-PET studies in the prediction of survival in patients with primary tumors. J Comput Assist Tomogr 1993;17(4):509–16.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med 1994;35:164–7.
Herholz K, Pietrzyk U, Voges J, Schroder R, Halber M, Treuer H, et al. Correlation of glucose consumption and tumor cell density in astrocytomas. A stereotactic PET study. J Neurosurg 1993;79:853–8.
Zasadny KR, Wahl RL. Standardized uptake values of normal fissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology 1993;189:847–50.
Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 1995;195:47–52.
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 1998;39:778–85.
Weber W, Bartenstein P, Gross MW, Kinzel D, Daschner H, Feldmann HJ, et al. Fluorine-18-FDG PET and iodine-123-IMT SPECT in the evaluation of brain tumors. J Nucl Med 1997;38:802–8.
Oriuchi N, Tomiyoshi K, Inoue T, Ahmad K, Sarwar M, Tokunaga M, et al. Independent thallium-201 accumulation and fluorine-18-fluorodeoxyglucose metabolism in glioma. J Nucl Med 1996;37:457–62.
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging 2002;29:176–82.
Alavi JB, Alavi A, Chawluk J, Kushner M, Powe J, Hickey W, et al. Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 1988;62:1074–78.
Inoue T, Shibasaki T, Oriuchi N, Aoyagi K, Tomiyoshi K, Amano S, et al. 18Fα-methyl tyrosine PET studies in patients with brain tumors. J Nucl Med 1999;40:399–405.
Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, et al. Synthesis of isomers of 18F labelled amino acid radiopharmaceutical: 2- and 3-L−18F-alpha-methyltyrosine using a separation and purification system. Nucl Med Commun 1997;18:169–75.
Ogawa T, Kanno I, Shishido F, Inugami A, Higano S, Fujita H, et al. Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 1991;32:197–202.
Shields AF, Grierson JR, Kozawa SM, Zheng M. Development of labeled thymidine analogs for imaging tumor proliferation. Nucl Med Biol 1996;23:17–22.
Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol 2000;27:143–56.
Mier W, Haberkorn U, Eisenhut M. [18F]FLT; portrait of a proliferation marker. Eur J Nucl Med Mol Imaging 2002;29:165–9.
Francis DL, Visvikis D, Costa DC, Arulampalam TH, Townsend C, Luthra SK, et al. Potential impact of [18F]3′-deoxy-3′-fluorothymidine versus [18F]fluoro-2-deoxy-D-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging 2003;30:988–94.
Seitz U, Wagner M, Neumaier B, Wawra E, Glatting G, Leder G, et al. Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3′-[18F]fluoro-3′-deoxythymidine ([18F]FLT) in pancreatic cancer cell lines. Eur J Nucl Med Mol Imaging 2002;29:1174–81.
Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3′deoxy-3′-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: correlation of [18F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002;8:3315–23.
Buck AK, Schirrmeister H, Hetzel M, Von Der Heide M, Halter G, Glatting G, et al. 3-deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 2002;62:3331–4.
Dittmann H, Dohmen BM, Kehlbach R, Bartusek G, Pritzkow M, Sarbia M, et al. Early changes in [18F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging 2002;29:1462–9.
Vijayalakshmi D, Belt JA. Sodium-dependent nucleoside transport in mouse intestinal epithelial cells. Two transport systems with differing substrate specificities. J Biol Chem 1988;263:19419–23.
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002;61(3):215–25.
Oh SJ, Mosdzianowski C, Chi DY, Kim JY, Kang SH, Ryu JS. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol 2004;31:803–9.
Di Chiro G. Positron emission tomography using [18F]fluorodeoxyglucose in brain tumors. A powerful diagnostic and prognostic tool. Invest Radiol 1987;22:360–71.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F]fluorodeoxyglucose and positron emission tomography. Neurology 1982;32:1323–9.
Tyler JL, Diksic M, Villemure JG, Evans AC, Meyer E, Yamamoto YL, et al. Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med 1987;28:1123–33.
Sasaki M, Kuwabara Y, Yoshida T, Nakagawa M, Fukumura T, Mihara F, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med 1998;25:1261–9.
Patronas P, Brooks RA, DeLaPaz RL, Smith BH, Kornblith PL, Di Chiro G. Glycolytic rate (PET) and contrast enhancement (CT) in human cerebral gliomas. Am J Neuroradiol 1983;4:533–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.J., Kim, J.S., Kim, J.H. et al. [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 32, 653–659 (2005). https://doi.org/10.1007/s00259-004-1742-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1742-3