Abstract
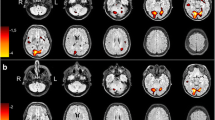
Statistical parametric mapping (SPM) quantification and analysis has been successfully applied to functional imaging studies of partial epilepsy syndromes in adults. The present study evaluated whether localisation of the epileptogenic zone (determined by SPM) improves upon visually examined single-photon emission tomography (SPET) imaging in presurgical assessment of children with temporal lobe epilepsy (TLE) and frontal lobe epilepsy (FLE). The patient sample consisted of 24 children (15 males) aged 2.1–17.8 years (9.8±4.3 years; mean±SD) with intractable TLE or FLE. SPET imaging was acquired routinely in presurgical evaluation. All patient images were transformed into the standard stereotactic space of the adult SPM SPET template prior to SPM statistical analysis. Individual patient images were contrasted with an adult control group of 22 healthy adult females. Resultant statistical parametric maps were rendered over the SPM canonical magnetic resonance imaging (MRI). Two corresponding sets of ictal and interictal SPM and SPET images were then generated for each patient. Experienced clinicians independently reviewed the image sets, blinded to clinical details. Concordance of the reports between SPM and SPET images, syndrome classification and MRI abnormality was studied. A fair level of inter-rater reliability (kappa=0.73) was evident for SPM localisation. SPM was concordant with SPET in 71% of all patients, the majority of the discordance being from the FLE group. SPM and SPET localisation were concordant with epilepsy syndrome in 80% of the TLE cases. Concordant localisation to syndrome was worse for both SPM (33%) and SPET (44%) in the FLE group. Data from a small sample of patients with varied focal structural pathologies suggested that SPM performed poorly relative to SPET in these cases. Concordance of SPM and SPET with syndrome was lower in patients younger than 6 years than in those aged 6 years and above. SPM is effective in localising the potential epileptogenic zone but does not provide additional benefit beyond SPET in presurgical assessment of children with intractable epilepsy. The impact of different pathologies on the efficacy of SPM warrants further study.

Similar content being viewed by others
References
Ho SS, Berkovic SF, Berlangieri SU, et al. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Ann Neurol 1995; 37:738–745.
Lawson JA, O’Brien T, Bleasel AF, et al. Utility of ictal SPECT in the diagnosis and management of intractable childhood epilepsy. Neurology 2000; 55:1391–1393.
O’Brien TJ, Mullan BP, Hauser MF, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localising the surgical seizure focus. Neurology 1998; 50:445–454.
Devous MD, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: a meta-analysis. J Nucl Med 1998; 39:285–293.
Kaminska A, Chiron C, Ville D. Ictal SPECT in children with epilepsy: comparison with intracranial EEG and relation to postsurgical outcome. Brain 2003; 126:248–260.
Stamatakis EA, Glabus MF, Wyper DJ, Barnes A, Wilson JTL. Validation of statistical parametric mapping (SPM) in assessing cerebral lesions: a simulation study. NeuroImage 1999; 10:397–407.
Van Laere KJ, Warwick J, Versijpt J, et al. Analysis of clinical brain SPECT data based on anatomic standardization and reference to normal data: an ROC-based comparison of visual, semiquantitative, and voxel based methods. J Nucl Med 2002; 43:458–469.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210.
Lee JD, Kim H, Lee BI, Kim OJ, Jeon TJ, Kim MJ. Evaluation of ictal brain SPET using statistical parametric mapping in temporal lobe epilepsy. Eur J Nucl Med 2000; 27:1658–1665.
Chang DJ, Zubal IG, Gottschalk C, et al. Comparison of statistical parametric mapping and SPECT difference imaging in patients with temporal lobe epilepsy. Epilepsia 2002; 43:68–74.
Davatzikos C, Henry HL, Herskovits E, Resnick SM. Accuracy and sensitivity of detection of activation foci in the brain via statistical parametric mapping: a study using a PET simulator. Neuroimage 2001; 13:176–184.
Koepp MJ, Richardson MP, Brooks DJ, et al. Cerebral benzodiaepine receptors in hippocampal sclerosis: an objective in vivo analysis. Brain 1996; 119:1677–1687.
Richardson MP, Koepp MJ, Brooks DJ, Fish DR, Duncan JS. Benzodiazepine receptors in focal epilepsy with cortical dysgenesis: an11C-flumazenil PET study. Ann Neurol 1996; 40:188–198.
Signorini M, Paulesu E, Perani D, et al. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and non-quantitative [18F]FDG PET: a clinical validation of statistical parametric mapping. Neuroimage 1999; 9:63–80.
Swartz BE, Thomas K, Simpkins F, Kovalik E, Mandlekern MM. Rapid quantitative analysis of individual18FDG-Pet scans. Clin Positron Imaging 1999; 2:47–56.
Van Bogaert P, Massager N, Tugendaft P, et al. Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. Neuroimage 2000; 12:129–138.
Lee DS, Lee JS, Kang KW, Jang SK, Chung J, Lee MC. Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on15O water PET, and [99mTc]-HMPAO SPECT. Epilepsia 2001; 42:1515–1522.
Hammers A, Koepp MJ, Labbe C, et al. Neocortical abnormalities of [11C]-flumazenil PET in mesial temporal lobe epilepsy. Neurology 2001; 56:897–906.
Richardson MP, Koepp MJ, Brooks DJ, Duncan JS. [11C]flumazenil PET in neocortical epilepsy. Neurology 1998; 51:485–492.
Kim YK, Lee D, Lee SK, Chung CK, Chung J, Lee MC. [18F]FDG PET in localisation of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med 2002; 43:1167–1174.
Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage 2000; 12:538–549.
Juhasz C, Behen ME, Muzik O, Chugani DC, Chugani HT. Bilateral medial prefrontal and temporal neocortical hypometaboilism in children with epilepsy and aggression. Epilepsia 2001; 42:991–1001.
Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ. Spatial registration and normalisation of images. Hum Brain Mapp 1995; 2:165–189.
Lawson JA, Nguyen W, Bleasel AF, et al. ILAE-defined epilepsy syndromes in children: correlation with quantitative MRI. Epilepsia 1998; 39:1345–1350.
Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989; 30:389–399.
Kwiatek R, Barnden L, Tedman R, Jarrett JC, Rowe C, Pile K. Regional cerebral blood flow in fibromyalgia: single-photon-emission computed evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum 2000; 43:2823–2833.
Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. New York: Thieme Medical, 1988.
Robb RA. Biomedical imaging, visualisation and analysis. New York: Wiley, 1999.
Armitage P, Berry G. Statistical methods in medical research. London: Blackwell Scientific; 1994:433–445.
Reiss AL, Abrams MT, Singer HS, Ross JL, Denkla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996; 119:1763–1774.
Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51:874–887.
Chiron C, Raynaud C, Maziere B, et al. Changes in regional cerebral blood flow during brain maturation in children and adults. J Nucl Med 1992; 33:696–703.
Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 2002; 15:870–878.
Van Laere KJ, Dierckx RA. Brain Perfusion SPECT: age- and sex-related effects correlated with voxel-based morphometric findings in healthy adults. Radiology 2001; 221:810–817.
Acknowledgments
The Brain Foundation (Australia) and Sydney Children’s Hospital Foundation provided financial support for this work. Tara Stevermuer (Centre for Health Service Development, University of Wollongong, NSW, Australia) and Sue Middleton (School of Mathematics, University of New South Wales, NSW, Australia) gave statistical support. Analyze software was supplied by Professor Richard A. Robb (Biomedical Imaging Resource, MAYO Foundation, Rochester, Minnesota, USA). We also thank Dr. Leighton Barnden (Department of Nuclear Medicine, Queen Elizabeth Hospital, Woodville, South Australia, Australia), for providing the adult control SPET images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruggemann, J.M., Som, S.S., Lawson, J.A. et al. Application of statistical parametric mapping to SPET in the assessment of intractable childhood epilepsy. Eur J Nucl Med Mol Imaging 31, 369–377 (2004). https://doi.org/10.1007/s00259-003-1366-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1366-z