Abstract
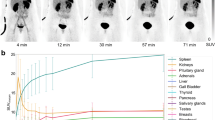
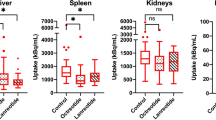
The pharmacokinetics and dosimetry of 86Y-DOTA0-d-Phe1-Tyr3-octreotide (86Y-SMT487) were evaluated in a phase I positron emission tomography (PET) study of 24 patients with somatostatin receptor-positive neuroendocrine tumours. The effect of amino acid (AA) co-infusion on renal and tumour uptake was assessed in a cross-over randomised setting. Five regimens were tested: no infusion, 4-h infusion of 120 g mixed AA (26.4 g l-lysine + l-arginine), 4 h l-lysine (50 g), 10 h 240 g mixed AA (52.8 g l-lysine + l-arginine) and 4 h Lys-Arg (25 g each). Comparisons were performed on an intra-patient basis. Infusions of AA started 0.5 h prior to injection of 86Y-SMT487 and PET scans were obtained at 4, 24 and 48 h p.i. Absorbed doses to tissues were computed using the MIRD3 method. 86Y-SMT487 displayed rapid plasma clearance and exclusive renal excretion; uptake was noted in kidneys, tumours, spleen and, to a lesser extent, liver. The 4-h mixed AA co-infusion significantly (P<0.05) reduced 86Y-SMT487 renal uptake by a mean of 21%. This protective effect was significant on the dosimetry data (3.3±1.3 vs 4.4±1.0 mGy/MBq; P<0.05) and was further enhanced upon prolonging the infusion to 10 h (2.1±0.4 vs 1.7±0.2 mGy/MBq; P<0.05). Infusion of Lys-Arg but not of l-lysine was more effective in reducing renal uptake than mixed AA. Infusion of AA did not result in reduced tumour uptake. The amount of 90Y-SMT487 (maximum allowed dose: MAD) that would result in a 23-Gy cut-off dose to kidneys was calculated for each study: MAD was higher with mixed AA co-infusion by a mean of 46% (10–114%, P<0.05 vs no infusion). In comparison with 4 h mixed AA, the MAD was higher by a mean of 23% (9–37%; P<0.05) with prolonged infusion and by a mean of 16% (2–28%; P<0.05) with Lys-Arg. We conclude that infusion of large amounts of AA reduces renal exposure during peptide-based radiotherapy and allows higher absorbed doses to tumours. The prolongation of the infusion from 4 to 10 h further enhances the protective effect on the kidneys.





Similar content being viewed by others
References
Lamberts SWJ, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocrine Rev 1991; 12:450–482.
Reubi JC. Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 1995; 36:1825–1835.
Krenning EP, Kooij PP, Bakker WH, Breeman WA, Postema PT, Kwekkeboom DJ, Oei HY, de Jong M, Visser TJ, Reijs AE, Lamberts SW. Radiotherapy with a radiolabeled somatostatin analogue [111In-DTPA-d-Phe1]-octreotide. A case history. Ann N Y Acad Sci 1994; 733:496–506.
Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, Stridsberg M, Lindemans J, Ensing G, Krenning EP. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med 2002; 32:110–122.
McCarthy KE, Woltering EA, Espanan GD, Cronin M, Maloney TJ, Anthony LB. In situ radiotherapy with111In-pentetreotide: initial observations and future directions. Cancer J Sci Am 1998; 4:94–102.
Stolz B, Smith-Jones P, Albert R, Albert R, Tolcsvai L, Briner U, Ruser G, Mäcke H, Weckbecker G, Bruns C. Somatostatin analogues for somatostatin-receptor-mediated radiotherapy of cancer. Digestion 1996; 57(Suppl 1):17–21.
Stolz B, Weckbecker G, Smith-Jones PM, Albert R, Raulf F, Bruns C. The somatostatin receptor-targeted radiotherapeutic [90Y-DOTA-d-Phe1, Tyr3]octreotide (90Y-SMT487) eradicates experimental rat pancreatic CA 20948 tumors. Eur J Nucl Med 1998; 25:668–674.
Otte A, Mueller-Brand J, Dellas S, Nitzsche EU, Herrmann R, Maecke HR. Yttrium-90-labelled somatostatin analogue for cancer treatment. Lancet 1998; 351:417–418.
Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, Haldemann A, Mueller-Brand J. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq90Y-DOTATOC. J Nucl Med 2002; 43:610–616.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, Caracciolo M, Mäcke HR, Chinol M, Paganelli G. Receptor-mediated radionuclide therapy with90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med in press; DOI 10.1007/s00259-002-1023-y.
Otte A, Hermann R, Heppeler A, Béhé M, Jermann E, Powell P, Maecke HR, Muller J. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999; 26:1439–1447.
de Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, Bakker WH, Smith C, Pauwels S, Krenning EP. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med 2002; 32:133–140.
Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with90Y-DOTATOC. Eur J Nucl Med 2001; 28:1552–1554
Akizawa H, Arano Y, Uezono T, Ono M, Fujioka Y, Uehara T, Yokoyama A, Akaji K, Kiso Y, Koizumi M, Saji H. Renal metabolism of111In-DTPA-d-Phe1-octreotide in vivo. Bioconjug Chem 1998; 9:662–670.
Hammond PJ, Wade AF, Gwilliam ME, Peters AM, Myers MJ, Gilbey SG, Bloom SR, Calam J. Amino acid infusion blocks renal tubular uptake of an indium-labeled somatostatin analogue. Br J Cancer 1993; 67:1437–1439.
Bernard BF, Krenning EP, Breeman WA, Rolleman EJ, Bakker WH, Visser TJ, Mäcke H, de Jong M.d-Lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med 1997; 38:1929–1933.
de Jong M, Rolleman EJ, Bernard BF, Visser TJ, Bakker WH, Breeman WA, Krenning EP. Inhibition of renal uptake of indium-111-DTPA-octreotide in vivo. J Nucl Med 1996; 37:1388–1392.
Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. EurJ Nucl Med 1998; 25:201–212.
Rolleman EJ, Valkema R, de Jong M, Kooij PPM, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med 2003; 30:9–15.
Rösch F, Herzog H, Stolz B, Brockmann J, Köhle M, Mühlensiepen H, Marbach P, Müller-Gärtner HW. Uptake kinetics of the somatostatin receptor ligand [86Y]-d-Phe1, Tyr3-octreotide ([86Y]SMT487) using positron emission tomography in non-human primates and calculation of radiation doses of the 90Y-labelled analogue. Eur J Nucl Med 1999; 26:358–366.
Förster GJ, Engelbach MJ, Brockmann J, Reber HJ, Buchholz HG, Mäcke HR, Rösch FR, Herzog HR, Bartenstein PR. Preliminary data on biodistribution and dosimetry for therapy planning of somatostatin receptor positive tumours: comparison of86Y-DOTATOC and 111In-DTPA-octreotide. Eur J Nucl Med 2001; 28:1743–1750.
Rösch F, Qaim SM, Stöcklin G. Production of the positron emitting radioisotope86Y for nuclear medical applications. Int J Appl Radiat Isot 1993; 44:677–681.
Wienhard K, Dahlbom M, Eriksson L, Michel C, Bruckbauer T, Pietrzyk U, Heiss WD. The ECAT EXACT HR: performance of a new high-resolution positron scanner. J Comput Assist Tomogr 1994; 18:110–118.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994; 13:601–609.
Walrand SH, Jamar F, Mathieu I, De Camps J, Lonneux M, Sibomana M, Labar D, Michel C, Pauwels S. Quantification in PET using isotopes emitting prompt single gammas: application to yttrium-86. Eur J Nucl Med in press. DOI 10.1007/s00259-002-1068-y.
ICRP Publication 41. Radiation protection. Nonstochastic effects of ionizing radiation. New York: Pergamon Press, 1991.
Pearson TC, Guthrie DL, Simpson J, Chinn S, Barosi G, Ferrant A, Lewis SM, Najean Y. Interpretation of measured red cell mass and plasma volume in adults. Expert Panel on Radionuclides of the International Council for Standardization in Haematology. Br J Haematol 1995; 89:748–756.
Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin MG, Orsi F, Maecke HR, Jermann E, Robertson C, Fiorenza M, Tosi G, Paganelli G. Biokinetics and dosimetry in patients administered with111In-DOTA-Tyr3-octreotide: implication for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 1999; 26:877–886.
de Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, Visser TJ, Jermann E, Béhé M, Powell P, Mäcke HR. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0, d-Phe1, Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997; 24:368–371.
Jonard P, Jamar F, Walrand S, Labar D, Mathieu I, Smith C, Kvols L, Krenning EP, Pauwels S. Effect of peptide amount on biodistribution of86Y-DOTA-Tyr3-octreotide (SMT487). J Nucl Med 2000; 41(Suppl):260P.
Mogensen CE, Sølling K. Studies on renal tubular protein reabsorption: partial and near complete inhibition by certain amino acids. Scand J Clin Lab Invest 1977; 33:477–486.
Acknowledgements
The expertise of Prof. C. Michel (Physics, PET Laboratory) and M. Cogneau (Nuclear Chemistry, PET Laboratory) was much appreciated. The authors thank P.P.M. Kooij (Erasmus MC, Rotterdam, Holland) for help in the dosimetry calculations.
Novartis Pharma Corp, East Hanover, N.J., financially supported this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamar, F., Barone, R., Mathieu, I. et al. 86Y-DOTA0-d-Phe1-Tyr3-octreotide (SMT487)—a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging 30, 510–518 (2003). https://doi.org/10.1007/s00259-003-1117-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1117-1