Abstract
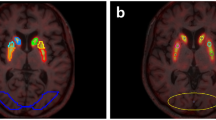
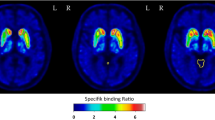
The objective of this study was to evaluate the reproducibility of123-FPCIT SPECT by using whole striatal region of interest (ROI) and subdivided ROI in normal controls (NC) and Parkinson’s disease (PD) patients.Methods: Ten NC and 6 PD received a SPECT scan for 6 hours postinjection of FPCIT. The distribution volume ratio (Rv) and specific-nonspecific tissue activity ratio (Rt) were measured as an outcome measure. The test/retest reproducibility of Rv and Rt was evaluated by calculating the test/retest difference, variability, and reliability.Results: There were no significant test/retest differences for any regions in either the NC or PD. The test/retest variability/reliability of Rv was 5.53 ± 4.12%/0.89 in NC, 4.50 ± 5.31%/0.99 in PD with whole striatal ROI, 4.29 ± 0.78%/ 0.94 ± 0.03 in NC, and 6.87 ± 1.23 %/0.98 ± 0.01 in PD with subdivided ROI. The test/retest variability/reliability of RT was 11.1 ± 10.4%/0.59 in NC, 7.84 ± 8.94%/0.95 in PD with whole striatal ROI, 11.9 ± 1.22%/0.65 ± 0.06 in NC, and 12.2 ± 4.00%/0.95 ± 0.03 in PD with subdivided ROI.Conclusion: Rv is highly reproducible and reliable compared with RT in both NC and PD as an outcome measure.
Similar content being viewed by others
References
Leenders KL, Salmon EP, Tyrrell P, Perani D, Brooks DJ, Sager H, et al. The nigrostriatai dopaminergic system as-sessedin vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s disease.Arch Neurol 1990; 47: 1290–1298.
Innis RB, Seibyl JP, Scanley BE, Lamelle M, Abi-Dargham A, Wallece E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease.Proc Natl Acad Sci USA 1993; 90: 11965–11969.
Frost JJ, Rosier AJ, Reich SG, Smith JS, Ehlers MD, Snyder SH, et al. Positron emission tomographic imaging of the dopamine transporters with11C-WIN 35,428 reveals marked declines in mild Parkinson’s disease.Ann Neurol 1993; 34: 423–431.
Guttman M, Burkholder J, Kish SJ, Hussey D, Wilson A, DaSilva J, et al. [11C]RTI-32 PET studies of the dopamine transporters in early dopa-naive Parkinson’s disease: implications for the symptomatic threshold.Neurology 1997; 48: 1578–1583.
Brücke T, Kohnhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I. SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT binding kinetics in human brain.J Neural Transm [GenSect] 1993; 94: 137–146.
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, ZeaPonce Y, et al. Decreased single-photon emission computed tomographic [123I]β-CIT striatal uptake correlates with symptom severity in Parkinson’s disease.Ann Neurol 1995; 38: 589–598.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AGM, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease.J Neurol Neurosurg Psychiatry 1997; 62: 133–140.
Booij J, Tissingh G, Winogrodzka A, Boer GJ, Stoof JC, Wolters EC, et al. Practical benefit of [123I]FP-CIT SPET in the demonstration of the dopaminergic deficit in Parkinson’s disease.Eur J Nucl Med 1997; 24: 68–71.
Lamelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi S, et al. Graphical, kinetic, and equilibrium analysis of mvivo [ 123]β-CYT binding to dopamine transporters in healthy human subjects.J Cereb Blood Flow Metab 1994; 14: 982–994.
Neumeyer JL, Wang S, Gao Y, Milius RA, Kula NS, Campbell A, et al. N-w- fluoroalkyl analogs of(lR)-2β- carbomethoxy-3β-(4-iodophenyl)-tropane (β-CIT): radiotracers for positron emission tomography and single photon emission computed tomography imaging of dopamine transporters.J Med Chem 1994; 37: 1558–1561.
Abi-Dargham A, Gandelman MS, DeErausquin GA, Zea-Ponce Y, Zoghbi SS, Baldwin RM, et al. SPECT imaging of dopamine transporters in human brain with iodine-123-fluoroalkyl analogs of J3-CIT.JNuclMed 1996; 37: 1129–1133.
Scheffel U, Lever JR, Abraham P, Parham KR, Mathews WB, Kopajtic T, et al.N-substituted phenyltropanes asin vivo binding ligands for rapid imaging studies of the dopamine transporter.Synapse 1997; 25: 345–349.
Kuikka JT, Bergström KA, Ahonen A, Hiltunen J, Haukka J, Lansimies E, et al. Comparison of iodine-123 labelled 2β-carbomethoxy-3β-(4-iodophenyl)tropane and 2β-carbomethoxy-3β-(4-iodophenyl)-A′-(3-fluoropropyl) nortropane for imaging of the dopamine transporter in the living human brain.Eur J Nucl Med 1995; 22: 356–360.
Booij J, Hamelaar JTGM, Speelman JD, de Bruin K, Janssen AGM, van Royen EA. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT.J Nucl Med 1999; 40: 753–761.
Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain.J Nucl Med 1993; 34: 609–613.
Vingerhoets FJG, Snow BJ, Schulzer M, Morrison S, Ruth TJ, Holden JE, et al. Reproducibility of fluorine-18-6-fluorodopa positron emission tomography in normal human subjects.J Nucl Med 1994; 35: 18–24.
Vingerhoets FJG, Schulzer M, Ruth TJ, Holden JE, Snow BJ. Reproducibility and discriminating ability of fluorine-18-6-fluoro-L-dopa PET in Parkinson’s disease.J Nucl Med 1996; 37: 421–426.
Seibyl JP, Lamelle M, van Dyck CH, Wallace E, Baldwin RM, Zoghbi S, et al. Reproducibility of iodine-123-β-CIT SPECT brain measurement of dopamine transporters.J Nucl Med 1996; 37: 222–228.
Ichise M, Ballinger JR, Vines D, Tsai S, Kung HF. Simplified quantification and reproducibility studies of dopamine D2-receptor binding with iodine-123-IBF SPECT in healthy subjects.J Nucl Med 1997; 38: 31–37.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality.Neurology 1967; 17: 427–442.
Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, ed.Quantification of Neurologic Deficit. Boston; Butterworths, 1989: 285–309.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases.J Neurol Neurosurg Psychiatry 1992; 55: 181–184.
Zea-Ponce Y, Baldwin RM, Laruelle M, Wang S, Neumeyer JL, Innis RB. Simplified multidose preparation of iodine-123-β-CIT: a marker for dopamine transporters.JNucl Med 1995; 36: 525–529.
Vines DC, Ivo BD, Ballinger JR, Ichise M. External radioactive reference markers in SPECT imaging of the dopamine system.J Nucl Med Technol 1999; 27: 112–116.
Snedecor GW, Cochran WG. Specific indices of interater reliability. In: Snedecor GW, Cochran WG, eds.Statistical Methods, 6th ed. Ames, IO; Iowa State University Press, 1989: 147–156.
Booij J, Habraken JBA, Bergmans P, Tissingh G, Winogrodzka A, Wolters EC, et al. Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease.J Nucl Med 1998; 39: 1879–1884.
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy.Ann Neurol 1990; 28: 547–555.
Gibb WR. Neuropathology of Parkinson’s disease and related syndromes.Neurol Clin 1992; 10: 361–376.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: pathophysiologic and clinical implications.N Engl J Med 1988; 318: 876–880.
Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Meumeyer J, et al. Comparative nigrostriatal dopaminergic imaging with iodine-123-βCIT-FP/SPECT and fluorine-18-FDOPA/PET.J Nucl Med 1996; 37: 1760–1765.
Seibyl JP, Marek K, Sheff K, Zoghbi S, Baldwin RM, Charney DS, et al. Iodine-123-β-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients.J Nucl Med 1998; 39: 1500–1508.
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AGM, van Royen EA, et al. Iodine-123-N-w)-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease.J Nucl Med 1998; 39: 1143–1148.
Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I]β-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease.Neurology 1996; 46: 231–237.
Vingerhoets FJG, Snow BJ, Lee CS, Schulzer M, Mak E, Calne DB. Longitudinal fluorodopa positron emission to-mographic studies of the evolution of idiopathic parkinsonism.Ann Neurol 1994; 36: 759–764.
Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson’s disease.Brain 1996; 119: 585–591.
Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, Kung HF. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT.J Nucl Med 1996; 37: 513–520.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsuchida, T., Ballinger, J.R., Vines, D. et al. Reproducibility of dopamine transporter density measured with123I-FPCIT SPECT in normal control and Parkinson’s disease patients. Ann Nucl Med 18, 609–616 (2004). https://doi.org/10.1007/BF02984583
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02984583