Visual Abstract
Abstract
This study measured the typical emitted radiation rate from the urinary bladder of PET patients after their scan and investigated simple methods for reducing the emitted radiation before discharge. Methods: The study included 83 patients (63 18F-FDG and 20 18F-NaF patients). Emitted radiation from the patients’ urinary bladder was measured with an ionization survey meter at a 1-m distance, presuming the urinary bladder to be the primary source of radiation. The measurements were taken at different time points after PET image acquisition: immediate (prevoid 1), voided (postvoid 1), after waiting 30 min in the uptake room while drinking 500 mL of water (prevoid 2), and voided again (postvoid 2). Results: For 18F-FDG patients, the reduction of emitted radiation due to drinking water and voiding alone from prevoid 1 to decay-corrected postvoid 2 was an average of 22.49% ± 7.48% (13.65 ± 3.42 μSv/h to 10.48 ± 2.37 μSv/h, P < 0.001). For 18F-NaF patients, the reduction was an average of 25.80% ± 10.03% (9.83 ± 2.01 μSv/h to 7.23 ± 1.49 μSv/h, P < 0.001). Conclusion: In addition to the physical decay of the radiotracers, using the biologic clearance properties resulted in a significant decrease of the emitted radiation in this study. Implementing additional water consumption to facilitate voiding with 30 min of wait time before discharging certain 18F-FDG and 18F-NaF patients who need to be in close contact with others, such as elderly, caregivers, and inpatients, might facilitate lowering their emitted radiation by an average of 22%–25% due to voiding, not counting in the physical decay that should add an additional 17% reduction.
PET imaging procedures have increased in the past few decades. The increased use of PET is attributed to multiple factors, including awareness of referring physicians and the emergence of a variety of tracers with numerous clinical applications (1). Further, the clinical indications of PET have expanded beyond oncology to include infection, inflammation, cardiovascular, brain and skeletal imaging. The first approved PET radiotracers by the U.S. Food and Drug Administration (FDA) and most widely used were 18F-FDG (18F-FDG) and 18F-sodium fluoride (18F-NaF) (2). Recently, the FDA has approved more PET radiotracers that are being used in clinical practice: 13N-ammonia in 2007, 18F-florbetapir in 2012, 18F-flutemetamol in 2013, 18F-florbetaben in 2014, 18F-fluciclovine in 2016, 68Ga-DOTATATE in 2016, 68Ga-DOTATOC in 2019, 18F-fluorodopa in 2019, 64Cu-DOTATATE in 2020, 18F-fluoroestradiol in 2020, and 68Ga-PSMA in 2020. Currently, there are additional PET radiotracers that are being evaluated in clinical trials and as investigational new drugs.
With the recent development of these PET radiotracers, there has been more attention given to the radiation exposure from PET patients after being discharged. Although radiation from medical use and nuclear medicine is overall safe (3,4), lowering radiation exposure from the patients to their caregivers or contacts is desirable. This is particularly important in special patient groups such as inpatients, who immediately return to their wards after imaging, and also for patients who require special assistance from a caregiver. There are a couple of studies that measured the emitted radiation from patients undergoing 68Ga-DOTATOC, 18F-fluorodopa, 18F-FDG, and 18F-fluciclovine scans (3,5).
Because most diagnostic studies before PET popularity were performed using 99mTc-labeled radiotracers, the discharge criteria for these studies are well-defined as there is minimal radiation exposure from the patients due to the 140 keV γ-emission and a 6-h half-life of a 99mTc radionuclide. On the other hand, PET radionuclides emit two 511-keV photons simultaneously, which are capable of more ionizing damage to their surroundings in comparison to 99mTc radionuclides. Therefore, both types of radiotracers cannot be treated equally and separate guidelines should be implemented for PET radiotracers. To date, however, there are no mandated release criteria for discharge of PET patients after completion of their scan.
Published articles have stated that most of the patients who underwent 18F-FDG scanning had emitted radiation exceeding or close to 20 μSv/h at the time of discharge (3–7). Muzaffar et al. stated that 97% of these patients had dropped the radiation exposure to below 20 μSv/h using simple interventions such as waiting half an hour after scanning and voiding before being discharged (5). This was, however, not the case with 18F-fluciclovine patients: only 25% of the patients had a drop of radiation exposure below 20 μSv/h after the same interventions. This observation is mainly because the imaging protocol and the biodistribution of 18F-fluciclovine are significantly different from those of 18F-FDG (5).
The objectives of this project were to determine the typical emitted radiation rate from the urinary bladder region of PET patients after the completion of 18F-FDG or 18F-NaF PET scans and to further investigate and validate the importance of simple interventions in an attempt to reduce the emitted radiation. These simple interventions may help in lowering the potential radiation exposure to close contacts without compromising the quality of images and at no additional cost.
MATERIALS AND METHODS
Patients undergoing PET scans in the nuclear medicine department for various clinical indications were asked to volunteer for this study. The study protocol was approved by the Kuwait University, Faculty of Medicine Ethical Review Committee as well as the Ministry of Health Ethical Review Committee. All the subjects signed a written informed consent form to participate in this study.
A total of 83 eligible patients consented to participate in the study. The study included patients undergoing PET scans using 18F-FDG or 18F-NaF. Patients who were bedridden, on kidney dialysis, with urine catheters, and under the age of 18 y were excluded from the study.
There were 63 patients (35 men and 28 women; mean age, 54.27 ± 15.14 y) who received a weight-based 18F-FDG dose of 5.18 MBq/kg ([0.14 mCi/kg]; range, 185–352 MBq [5–9.5 mCi]) (Table 1). After injection, the patients had an approximately 60-min uptake time followed by a whole-body PET/CT acquisition of about 15–20 min (Gemini TF 64 slice PET/CT; Philips). Each patient’s equivalent dose rate was then measured with an ionization survey meter (GM Detector, model IA-V2; International Medcom) at 1 m immediately after the completion of the PET scan. On the basis of the institutional guidelines, the ionization survey meter is calibrated every 6 mo. For distance consistency, 2 tape marks were placed on the floor of the uptake rooms at a 1-m distance. Patients were asked to stand by one of the tape marks on the floor with the technologist on the other tape mark. Presuming the urinary bladder is the primary source of activity emitted from the patient, the radiation emissions from the urinary bladder were recorded. The bladder was assumed to be in its normal location in the pelvis. The survey meter was held by the technologist at the height of the patient’s urinary bladder. The measurements were recorded after the radiation reading became steady on the ionization survey meter. After the initial radiation measurement (prevoid 1), the patients were then asked to void, and another measurement (postvoid 1) was recorded. Then the patients were given 500 mL of water to drink while waiting for 30 min in the uptake room and instructed not to void during this period. As per the study protocol, the patients waited in their individual uptake rooms and did not come into contact with anyone during this time. Additional measurements were recorded after the 30-min wait (prevoid 2), and finally, the patients were asked to void again for a last measurement (postvoid 2) before being discharged from the department. The average stay of the patients in the department during this study was 139 ± 16 min (range, 86–177 min) from the time of 18F-FDG administration until the time of the postvoid 2 measurement.
Patient Demographic Data
For 18F-NaF, 20 patients (8 men and 12 women; mean age, 57.55 ± 18.69 y) were eligible and agreed to participate in this study (Table 1). These patients received a weight-based dose of 5.18 MBq/kg ([0.14 mCi/kg] range, 186–376 MBq [5.02–10.17 mCi]). The emitted radiation was measured in a manner similar to that for 18F-FDG patients. The average stay of the patients in the department during this study was 168 ± 15 min (range, 140–191 min) from the time of 18F-NaF administration until the time of the postvoid 2 measurement.
Statistical Analysis
The IBM Statistical Package for Social Sciences (version 23; SPSS-Inc.) was used to perform all statistical analyses. Group statistics, providing basic information about group comparisons, including the sample size (n), mean, and SD, were calculated and presented as mean ± SD. The independent-samples t test was conducted to compare the means between groups to determine statistical significance.
The data were analyzed on the basis of different categories, including sex, body mass index (BMI), and age. In the sex category, there were 35 men and 28 women for the 18F-FDG group and 8 men and 12 women in the 18F-NaF group. In the BMI category, the patients were grouped according to World Health Organization classifications: a normal group, from 18.5 to 24.9; an overweight group, from 25 to 29.9; and an obese group, with a BMI of 30.0 and higher (8). There were 13 normal, 22 overweight, and 28 obese 18F-FDG patients, and there were 5 normal, 5 overweight, and 10 obese 18F-NaF patients. The age category included a youth group of 18–24 y old, an adult group of 25–64 y old, and a senior group of 65 y and older. For the 18F-FDG patients, there were 2 in the youth group, 41 in the adult group, and 20 in the senior group. For the 18F-NaF patients, there were 2 in the youth group, 8 in the adult group, and 10 in the senior group.
RESULTS
18F-FDG Patients
For 18F-FDG patients, the average decrease of emitted radiation rate from prevoid 1 to postvoid 1 was 10.05% ± 6.54% (13.69 ± 3.42 μSv/h to 12.16 ± 2.74 μSv/h, P = 0.008) as illustrated in Figure 1. The average decrease from prevoid 2 to postvoid 2 was 12.08% ± 6.02% (9.87 ± 2.18 μSv/h to 8.67 ± 1.96 μSv/h, P = 0.001). The average reduction of emitted radiation due to drinking water and voiding from prevoid 1 to decay-corrected postvoid 2 was 22.49% ± 7.48% (13.65 ± 3.42 μSv/h to 10.48 ± 2.37 μSv/h, P < 0.001).
Exposure rates (μSv/h at 1 m) from 18F-FDG patients (n = 63) at different time points.
In the sex category, the difference in the overall reduction of the emitted radiation between the men and women was not statistically significant (Fig. 2). In the BMI category, the difference in an overall reduction of the emitted radiation between the normal, overweight, and obese patient groups was not statistically significant (Fig. 2). For the grouping based on age, the difference in overall reduction of the emitted radiation between the youth, adult, and senior groups was not statistically significant (Fig. 2).
Comparison of overall reduction of emitted radiation from voiding after decay correction of categorized 18F-FDG patients with corresponding P values. P values indicate no statistical significance between different patient categories regarding effect of decay-corrected void. Youth group has only 2 patients, therefore, P value of this group was not calculated.
18F-NaF Patients
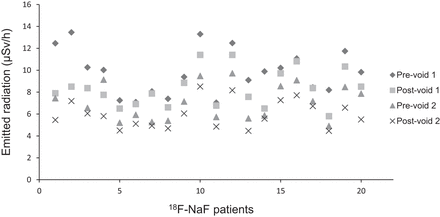
For 18F-NaF patients, the average decrease of emitted radiation rate from prevoid 1 to postvoid 1 was 13.33% ± 11.26% (9.83 ± 2.01 μSv/h to 8.32 ± 1.63 μSv/h, P = 0.011) as illustrated in Figure 3. The average decrease from prevoid 2 to postvoid 2 was 15.64% ± 8.17% (7.08 ± 1.58 μSv/h to 5.98 ± 1.24 μSv/h, P = 0.012). The average reduction of emitted radiation rate due to drinking water and voiding from prevoid 1 to decay-corrected postvoid 2 was 25.80% ± 10.03% (9.83 ± 2.01 μSv/h to 7.23 ± 1.49 μSv/h, P < 0.001).
Exposure rates (μSv/h at 1 m) from 18F-NaF patients (n = 20) at different time points.
In the sex category, the difference in the overall reduction of the emitted radiation between the men and women was not statistically significant (Fig. 4). In the BMI category, the difference in an overall reduction of the emitted radiation between the normal, overweight, and obese patient groups was not statistically significant (Fig. 4). For the category based on age, the overall reduction in the emitted radiation between the youth, adult, and senior patient groups was not statistically significant (Fig. 4).
Comparison of overall reduction of emitted radiation from voiding after decay correction of categorized 18F-NaF patients with corresponding P values. P values demonstrate that there was no statistical significance between different categories regarding effect of decay-corrected void. Youth group has only 2 patients, therefore, P value of this group was not calculated.
DISCUSSION
Nuclear medicine departments routinely perform both diagnostic and therapeutic procedures using a variety of radionuclides with different types and energies of emitted radiations. Most performed procedures in nuclear medicine are diagnostic radionuclide imaging. Extensive work has been undertaken for the reduction of radiation exposure to patients and nuclear medicine staff (9–13). It has been well reported that patient radiation exposure from nuclear medicine is overall safe and might be beneficial in some cases (3,4). Consequently, nuclear medicine practice incorporates important principles for the reduction of the radiation dose, such as As Low As Reasonably Achievable (ALARA) principle. The nuclear medicine staff are trained to handle all types of radioactivity, keeping in mind the time, distance, and shielding principles to minimize radiation exposure. In addition, the radiation exposure of the nuclear medicine staff is continuously monitored to ensure that the allowed radiation dose limits are not exceeded.
Unlike the radiation exposure to patients and nuclear medicine staff, the radiation exposure from the PET patients at the time of discharge has not been extensively addressed. The goal of our study was to determine the typical emitted radiation rate from the urinary bladder region of patients after the completion of 18F-FDG or 18F-NaF PET scans and to evaluate simple, noninvasive interventions aimed at reducing radiation exposure to close contacts and caregivers from the discharged PET patients using both the physical half-life and the biologic half-life. The physical half-life is the time during which an initial activity of a radionuclide is reduced to one half by physical decay (14). Biologic half-life is the time by which one half of the administered dose is eliminated via biologic processes such as urinary and fecal excretion (14). Effective half-life is calculated on the basis of both the physical half-life and the biologic half-life for each radiotracer. It is defined as the time required for an initial administered dose to be reduced to one half due to both the physical decay and the biologic elimination of the radiotracer (14).
The 2 PET tracers investigated in this study are eliminated via the kidneys, with the urinary bladder being the organ with the highest radiation-absorbed dose (15–19). However, each radiotracer has a different biologic half-life. About 21% of 18F-FDG is cleared in urine approximately 2 h after administration (15). For 18F-NaF, about 20% is cleared in urine within the first 2 h (16,17). Both 18F-FDG and 18F-NaF are labeled with the same radionuclide, that is, 18F, which has a physical half-life of 110 min. The shorter physical and biologic half-lives of PET radiotracers allow for faster elimination, and hence presumably implementing simple interventions based on these properties before patient discharge may be potentially advantageous. Having the patient wait for a certain period of time before being discharged is based on the decay property of the radionuclide. As for voiding, the concept of biologic half-life is important, and this is achieved by ensuring that the patient is well hydrated during the uptake time and before discharge.
Our data show that a simple precautionary measure of making the patients void before discharge reduces the emitted radiation by a mean of about 10% for 18F-FDG and 13% for 18F-NaF. Waiting an additional 30 min while drinking water resulted in an additional reduction of the emitted radiation by 12% and 16% for 18F-FDG and 18F-NaF, respectively, because of revoiding. From another perspective, a 30-min exposure dose at a 1-m distance would be around 6.83 and 4.92 μSv from 18F-FDG and 18F-NaF patients, respectively, at the standard time of discharge. This radiation exposure dose would drop to 4.33 and 2.99 μSv from 18F-FDG and 18F-NaF patients, respectively, after the simple steps outlined in this study. This decrease might be of benefit in patients who need to be in close contact with a caregiver. These include elderly patients and young patients as well as their mothers, particularly mothers who are nursing or have young children who would not comply with the instruction to maintain a safe distance. Also, there are other patients who do not have the luxury of separate rooms or bathrooms in their homes, and they may benefit from the extra time in the department before discharge. This might also be beneficial for inpatients who will be returning back to the ward immediately after completing the scan and potentially exposing other patients and nursing staff to unnecessary radiation.
A previously published article by Muzaffar et al. aimed at introducing simple methods to reduce radiation exposure rates to the public from 18F-FDG PET/CT patients (5). They used 18F-FDG doses of 370–740 MBq (10–20 mCi), and our patients were injected with 18F-FDG doses of 185–352 MBq (5–9.5 mCi). Muzaffar et al. reported that about 75% of their patients leave the imaging facility with emitted radiation exceeding 20 μSv/h (2 mR/h) (3). Only 3% of our patients would have left the department with emitted radiation exceeding 20 μSv/h because they were injected with lower doses than the patients in the study by Muzaffar et al. Our data also showed that the overall emitted radiation reduction from both 18F-FDG and 18F-NaF was not affected by the patients’ sex, BMI or age, as the P values showed no statistical significance.
Patient preparation before the scan may play an important role in decreasing the radiation dose. Good hydration and voiding have always been advised and recommended before, during, and after the scan in the patients’ instructions but are not usually reenforced (20–23). This is mainly recommended to accelerate the clearance of the background blood-pool activity to improve the image quality and decrease the radiation dose to the patient (20–23). In addition to these benefits, based on our decay-corrected data, the biologic clearance permitted the decrease of emitted radiation of an average of 22.49% for 18F-FDG, which is equivalent to 40 min of 18F decay time, and 25.80% for 18F-NaF, which is equivalent to 47 min of 18F decay time. Therefore, good hydration assisted in significantly decreasing the emitted radiation from the patients to their close contacts.
The decay property of 18F will always result in a reduction of 17% of the emitted radiation from the patients when they wait 30 min. However, the drop of radiation due to decay cannot be measured accurately from our collected data because the radiotracer is continuously circulating in the patients’ body between postvoid 1 and prevoid 2 measurements as accumulation of the radiotracer is taking place in the urinary bladder. Therefore, this value was not calculated from our data as it is not feasible.
During this study, none of the staff was exposed to additional radiation since the department has shielded uptake rooms in the PET suite. Each patient stayed comfortably in their individual uptake room without exposing any of the nuclear medicine staff to additional radiation. We can accommodate the use of these rooms even if there is a busy schedule. However, the logistics vary from one hospital to another and this is outside the scope of this article.
The lower-than-expected number of patients was a limitation, as most of the eligible patients did not consent to be a part of this study. In addition, the renal function tests of most patients were not available due to logistical issues. Therefore, it was not feasible to study the relationship between renal function and its effect on emitted radiation rates. Also, using a whole-body radiation counter would have provided a more accurate measurement, but unfortunately, this was not available in our institution. However, on the basis of the collected data from the 2 different PET tracers, it was obvious that using both physical decay and biologic elimination properties had a significant impact on lowering the emitted radiation from the patients. There should not be major logistical issues to implement these steps at the nuclear medicine department since it will be based on individual cases.
CONCLUSION
With the increasing use of PET in clinical practice and the approval of new PET radiotracers, the emitted radiation from the discharged PET patients has been of interest. Use of the biologic half-life properties of radiotracers demonstrated a significant impact on lowering the emitted radiation rate from PET patients. Requesting the patient to consume additional water after the completion of the scan will facilitate voiding with 30 min of wait time before being discharged, which will be of benefit to certain PET patients such as the elderly, caregivers, and inpatients who need to be in close contact with others. In addition to the possible reduction of emitted radiation rates an average of 22%–25% due to voiding, there is an additional 17% reduction due to decay of the radioactivity during this time.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Will undertaking simple steps with the PET patients before their discharge from the department significantly reduce the emitted radiation to their close contacts?
PERTINENT FINDINGS: In a prospective study of patients undergoing PET scans, the emitted radiation from their urinary bladders were measured after completing the exam (prevoid 1), voiding (postvoid 1), waiting 30 min while drinking water (prevoid 2), and voiding again (postvoid 2). Overall, voiding in this study resulted in an average decrease of emitted radiation rate of 22.49% for 18F-FDG and 25.80% for 18F-NaF, in addition to a fixed 17% decrease from the physical decay of 18F radiotracers after 30 min of wait time.
IMPLICATIONS FOR PATIENT CARE: Following simple steps after the completion of the PET scan will significantly decrease the emitted radiation from the PET patients to their close contacts.
Footnotes
Published online Apr. 19, 2022.
REFERENCES
- Received for publication October 2, 2021.
- Accepted for publication March 21, 2022.