Abstract
Quantitative myocardial PET perfusion requires decay correction (DC) of the dynamic datasets to ensure that measured activity reflects true physiology and not radiotracer decay or frame duration. DC is typically performed by the PET camera system, and the exact algorithm is buried within the settings and assumed to be correct for quantitative perfusion data. For quantitative myocardial perfusion, sequential dynamic images should be decay-corrected to the activity at the midpoint of the first scan in the sequence. However, there are different DC algorithms that can be implemented depending on the needs and expertise of the laboratory. As such, before quantitative myocardial perfusion is performed, the DC technique of a camera system should be tested.
- image processing
- PET
- decay correction
- myocardial blood flow
- positron emission tomography
- arterial input function
Quantification of absolute myocardial blood flow (MBF) with PET has become mainstream and is now reimbursed through the Centers for Medicare and Medicaid services. The literature describing the technical requirements for accurate reproducible MBF is extensive (1–4). An essential but commonly overlooked function is decay correction (DC), particularly for older or refurbished 2-dimensional (2D) or 3-dimensional (3D) scanners already in use. Since these are assumed to be working properly for quantification of MBF, the literature offers little information for practical, simple testing in order to assess the DC of an installed PET scanner or an older refurbished scanner under consideration for purchase.
The goals of this article are 2-fold. The first is to explain the rationale and methods for DC for assessment of MBF. The second is to report an easy method by which technologists can assess DC when there is no onsite physicist or technical expert.
BASICS OF QUANTITATIVE PET PERFUSION
Measuring MBF requires 2 primary data: the concentration of radiotracer in the arterial blood over time (also known as arterial input [A0] or the early blood pool phase) and the concentration of radiotracer in the myocardium (also known as myocardial uptake [M] or the late myocardial phase). For all PET scanners, radionuclides, acquisition protocols, flow models, and list mode or binned data, accurate DC of these datasets is essential but often buried from the end user and assumed to be correct for MBF studies. Different DC algorithms may be appropriate for different types of imaging (brain, cardiac, oncology), half-lives of the radiotracer, or questions asked (drug metabolism, scanner performance, MBF) (5). Consequently, each established PET scanner that is to be used for MBF should be checked for correct DC.
RATIONALE FOR DC ON QUANTIFICATION OF MBF
Why is DC necessary for accurate and precise quantification of MBF? Although the various kinetic models correct for partial-volume loss, spillover, extraction, and exit from myocardium, in the simplest conceptual form MBF derives from the ratio of M to A0 (6,7).
The A0 images quantify the change in concentration of radiotracer in the blood pool over time, before myocardial extraction, due to dilution by circulating blood and lung volume after intravenous injection. The M images quantify the average concentration of radiotracer trapped in the myocardium after clearance from the blood pool. As a potassium analog, 82Rb does not leak from myocardium except in cases of severe cell injury wherein intracellular potassium is not maintained. The slow leakage of 13N ammonia from myocardium after initial uptake is accounted for in its flow model. The simplistic inverse relationship between MBF and A0 shows how erroneous MBF may be due to too high or too low an A0 or M, all of which may be due to incorrect DC.
For quantitative perfusion studies, the radiotracer concentrations of A0 and M should be dictated solely by physiology and not by radiotracer decay, image duration, or acquisition parameters (number of frames in an acquisition). If A0 images are not decay-corrected, the downstream impact would lead to erroneously reduced A0 and thus falsely high MBF. In addition, there is also a differential impact of incorrect DC between rest and stress datasets, thereby causing errors in stress mL/min/g and coronary flow reserve over and above the physiologic effects of cardiac output, heart rate, and blood pressure during stress compared with rest.
With the short-lived 82Rb having rapid decay over 75 s, an erroneous DC will particularly degrade quantitative data in both A0 and M phases. Because of the physiologic rapidly changing high blood concentrations of the A0 phase, A0 data are more prone to cause errors in MBF than are M data. The impact of incorrectly reduced A0 and M data will yield inaccurate elevation in absolute MBF ranging from 10% to 40% (1,6,8).
UNDERSTANDING PET SCANNER DC
As a thought experiment, imagine a radiotracer X with a half-life approaching infinity. If 185 MBq (5 mCi) of X, as measured in a dose calibrator, is placed in a beaker filled with exactly 500 cm3 of H2O, the concentration of X would be 0.37 MBq/cm3 (10 μCi/cm3) at time 0. Because this imaginary radiotracer’s half-life is infinite, there is essentially a stable concentration of 0.37 MBq/cm3 (10 μCi/cm3) over time. For each cm3, the beaker is emitting 3.70 × 105 disintegrations per second, or 0.37 MBq (10 μCi). If this beaker is now placed into an ideal camera system that captures every disintegration and an image is acquired over 10 s, what is the camera doing? In a sample volume of 1 cm3, the camera receives 3.70 × 105 counts in the first second and 3.70 × 105 counts in each second afterward. Therefore, over 10 s, the scanner has received 3.7 × 106 counts. The units are integrated activity multiplied by time (count/cm3 × s). The total cumulative activity increases over time depending on the count/s coming from the beaker sample volume. This total cumulative integrated activity divided by the total image duration gives the average count/s emitted by the beaker sample volume. In this example, 3.70 × 106 counts/cm3/s × s divided by 10 s gives the original target concentration of 3.70 × 105 counts/s per cm3 or 0.37 MBq/cm3 (10 μCi/cm3).
However, in the real clinical world—where decay occurs rapidly, scanners do not capture all disintegrations, and biologic processes influence the concentration of radiotracer—how does the camera operate such that the measured activity reflects the true activity of the biologic process? The main function of DC is to recalculate measured activity for each time frame into values that would be measured if decay did not occur, thus ensuring accurate arterial and myocardial activity essential for MBF.
The mathematic description of radioactive decay is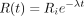 (Eq. 1)where
(Eq. 1)where 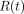 is the amount of radiotracer at time t,
is the amount of radiotracer at time t, 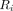 is the initial amount of radiotracer at the start of the scanning period, and λ is the decay constant of the radiotracer. With regard to quantitative perfusion with PET, there are 2 methodologic predicaments that can be deduced from this equation. First,
is the initial amount of radiotracer at the start of the scanning period, and λ is the decay constant of the radiotracer. With regard to quantitative perfusion with PET, there are 2 methodologic predicaments that can be deduced from this equation. First,  is not actually measured. The PET scanner accumulates and integrates counts over a time interval. Thus,
is not actually measured. The PET scanner accumulates and integrates counts over a time interval. Thus,  equals
equals 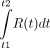 , where t1 and t2 are the time duration of the scan or frame. Second,
, where t1 and t2 are the time duration of the scan or frame. Second, 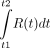 is influenced by factors other than decay, such as myocardial extraction and retention. In other words, the activity of radiotracer in a scanner region of interest (ROI) will depend on the duration of the time interval, the decay of the radiotracer, and any biologic process that removes or adds radiotracer from the ROI. Hence, to accurately measure the activity of A0 and M, decay of the radiotracer must be corrected for the duration of the scanning intervals.
is influenced by factors other than decay, such as myocardial extraction and retention. In other words, the activity of radiotracer in a scanner region of interest (ROI) will depend on the duration of the time interval, the decay of the radiotracer, and any biologic process that removes or adds radiotracer from the ROI. Hence, to accurately measure the activity of A0 and M, decay of the radiotracer must be corrected for the duration of the scanning intervals.
Many PET scanners offer different DC options to correct sequential images relative to the activity at some point during the scan (5,9,10). For dynamic processes or for imaging PET tracers with half-lives shorter than the acquisition period, the midpoint of the first scan is used (5). This correction confirms that any subsequent change in activity in later sequences is due to biologic change and not to image duration, interval between images, or radiotracer decay.
As an example, a beaker containing 470 cm3 of H2O is mixed with 30 cm3 of 370-MBq (10 mCi) 82Rb (half-life, 76 s), yielding 0.74 MBq/cm3 (20 μCi/cm3) at time zero as confirmed with a dose calibrator. If serial images are captured every 20 s for 3 frames, frames 1, 2, and 3—because of decay and calculated using the equation  —have an actual average concentration of 0.666, 0.562, and 0.470 MBq/cm3 (18.3, 15.2, and 12.7 μCi/cm3), respectively. However, the camera system should decay-correct frames 2 and 3 using a reference time of 10 s into the scan (half the interval of the first frame). Corrected for decay, frames 2 and 3 will have an average concentration of approximately 0.666 MBq/cm3 (18.3 μCi/cm3) and all 3 frames should yield nearly identical concentrations, despite the fact that counts/s and concentrations are decreasing with time. The difference between the concentration at time 0 and the actual measured average concentration of the first frame is due to decay during the 20-s acquisition and the lag time of the first few seconds of the scanner, hence the rationale for using the midpoint as the reference (5).
—have an actual average concentration of 0.666, 0.562, and 0.470 MBq/cm3 (18.3, 15.2, and 12.7 μCi/cm3), respectively. However, the camera system should decay-correct frames 2 and 3 using a reference time of 10 s into the scan (half the interval of the first frame). Corrected for decay, frames 2 and 3 will have an average concentration of approximately 0.666 MBq/cm3 (18.3 μCi/cm3) and all 3 frames should yield nearly identical concentrations, despite the fact that counts/s and concentrations are decreasing with time. The difference between the concentration at time 0 and the actual measured average concentration of the first frame is due to decay during the 20-s acquisition and the lag time of the first few seconds of the scanner, hence the rationale for using the midpoint as the reference (5).
TESTING DC
In practice, DC can easily be tested using a simple protocol that requires a graduated cylinder, dose calibrator, 500-cm3 beaker, and stopwatch. A solution of precise volume and dose of radiotracer is created in the beaker. An aliquot is withdrawn and inserted into a dose calibrator, and the beaker is positioned in the scanner. The scanner is started at the very moment that the dose calibrator measures the activity of the aliquot at time 0. The beaker is then scanned over the duration in which significant decay occurs. For 82Rb, measurement from 3.5 to 7 min is adequate; for 13N, from 10 to 15; and for 18F, from 40 to 60 min. The acquisition should allow for several frames to be created over the duration of the scan. For established 2D or 3D scanners acquiring in list mode, the frames can be created after the acquisition; however, for non–list-mode cameras, the protocol should be prespecified. Most modern 3D scanners correct for decay automatically as the data are acquired and likely do not require such testing for DC. All images should also be attenuation-corrected. After the attenuation-corrected frames are created, ROIs are drawn around the radiotracer activity for each frame and the average concentration is recorded by the scanner. If DC is set up correctly for MBF studies, the average concentration should be nearly identical in each frame and fall within a ±3% window from the first frame. Furthermore, the ratio of the calculated concentration of the first frame (based on the dose calibrator) to the measured concentration can be determined. This ratio should be about 1.00 ± 10% if the timing of the testing protocol was performed accurately; has accurate random, scatter, and dead-time corrections; and is calibrated correctly for the radiotracer being imaged. If not, further testing of other scanner function or calibration is needed. Distinct protocols for 18F, 13N, and 82Rb that can be performed by one person, in addition to worksheets for 18F, 13N, and 82Rb, can be found in the supplemental materials (available at http://jnm.snmjournals.org).
CASE EXAMPLES
Figures 1⇓–3 illustrate beaker tests performed on an Attrius 2D PET scanner (Positron) that demonstrates accurate DC of 18F, 13N, and 82Rb in a 500-mL beaker with a dose calibrator as the reference standard.
Decay of 18F in 500-cm3 beaker. A detailed 18F protocol (“Fluorine Decay Correction.docx”) and worksheet (“F18 Decay Correction Worksheet.xlsx”) can be found in the supplemental materials. With syringe, 153.18 MBq of 18F were placed in beaker containing 500 cm3 of H2O. Accounting for residual activity in transfer syringe and elapsed time between dose calibrator measurement and start of scan, concentration of 18F at start of scan was 0.270 MBq/cm3. Scanner performed 2,400-s (40 min) list-mode acquisition. Twenty-four hours later, after all activity decayed, attenuation scanning was performed and 5 serial frames were generated at intervals of 300, 300, 300, 600, and 900 s. ROIs were placed, avoiding beaker boundaries. Activity at time t equaled initial activity × e(−0.693 × t/(half-life of radiotracer). Therefore, with starting activity of 0.270 MBq/cm3, expected activity at 150 s into scan (midpoint of first frame) is 0.266 MBq/cm3. Half-life of 18F is 6,600 s, and 0.270 MBq/cm3 × e (−6.93 × 150 s/6,600s) = 0.266 MBq/cm3. Concentration of ROI in first frame was 0.266 MBq/cm3, which is 0.1% bias from dose calibrator. Concentrations in each subsequent frame were nearly identical to first frame, with biases all within 3% window. There are several conclusions. First, scanner decay-corrects activity to midpoint of first frame. Second, scanner also corrects for duration of each frame, giving activity in MBq/cm3. Third, in biologic systems, only variation in quantitative activity after first frame would be due to physiologic changes and not imaging timing, duration, or decay. Bias from dose calibrator of first frame, also known as efficiency, is inconsequential to measurements of MBF as it cancels out in numerator and denominator of flow equations (6). Bias does inform us on whether test was performed with accurate timing, random, scatter, and dead-time corrections and also on whether camera system was internally calibrated for isotope against standard. If timing of beaker decay test is not precise or camera has not been calibrated, measured concentration could be significantly different from dose calibrator; however, if DC is performed correctly, bias of subsequent frames will be uniform.
Decay of 13N in 500-cm3 beaker. A detailed 13N protocol (“Nitrogen Decay Correction.docx”) and worksheet (“13N Decay Correction Worksheet.xlsx”) can be found in the supplemental materials. Similar to Figure 1, 348.91 MBq of 13N were placed a beaker containing precisely 500 cm3 of H2O. Scanner performed 600-s (10 min) list-mode acquisition. Two hours later, after all activity decayed, attenuation scanning was performed and 5 serial frames were generated at intervals of 60, 60, 120, 120, and 240 s. Calculations and measurements were as shown in Figure 1. There are several conclusions. First, scanner decay-corrects activity to midpoint of first frame. Second, scanner also corrects for duration of each frame, giving activity in MBq/cm3. Third, in biologic systems, only variation in quantitative activity after first frame would be due to physiologic changes and not imaging timing, duration, or decay.
Decay of 82Rb in 500 cm3 beaker. A detailed 82Rb protocol (“Rubidium Decay Correction.docx”) and worksheet (“82Rb Decay Correction Worksheet.xlsx”) can be found in the supplemental materials. Similar to Figures 1 and 2, 1,073 MBq of 82Rb were placed in a beaker with a total volume precisely 500 cm3 (H2O plus 82Rb eluate). Scanner performed 720-s (12 min) list-mode acquisition. Ten minutes later, after all activity decayed, attenuation scanning was performed and 3 serial frames were generated at intervals of 120, 300, and 300 s. Calculations and measurements were as shown in Figure 1. There are several conclusions. First, scanner decay-corrects activity to midpoint of first frame. Second, scanner also corrects for duration of each frame, giving activity in MBq/cm3. Third, in biologic systems, only variation in quantitative activity after first frame would be due to physiologic changes and not imaging timing, duration, or decay.
To investigate a refurbished 2D camera system for which absolute flow values were thought to be erroneously high, a DC beaker test using 18F in a 500-cm3 volume was performed. Figure 4 illustrates the results of inaccurate DC by the scanner. To confirm that this problem was not unique to the individual camera, a different camera from the same vendor was also tested and yielded similar results. Figure 5 demonstrates the relative and quantitative perfusion data from the refurbished 2D camera using the factory-installed incorrect DC algorithm and after the vendor corrected the DC algorithm. The relative images were normal and demonstrated no significant differences between the correct and incorrect DC algorithms. However quantitative perfusion data were about 30% higher at rest and about 55% higher at stress with incorrect DC, because of a falsely reduced A0. Besides the obvious difference in MBF values, there are several conclusions that can be made. First, relative perfusion imaging is not impacted, and therefore incorrect DC can easily go unnoticed. Second, both sets of MBF values are physiologically plausible and therefore erroneous MBF values can easily go unnoticed, thus skewing site-specific reference datasets to higher MBF values. Third, prior testing on the performance of various camera systems did not specifically confirm correct DC but relied on “routine clinical practice at each institution” (3). Hence, although a camera’s performance with regard to peak counts, dead time, scatter, and randoms may be acceptable for MBF studies, inaccurate DC will still yield erroneous quantitative data. Finally, there is the possibility that clinicians or researchers who have older refurbished PET cameras with incorrect decay algorithms are making clinical decisions with erroneous MBF values.
Similar to Figures 1--3, decay beaker testing, using 18F, was performed on 2D refurbished PET camera when there was concern about accuracy of MBF data. Scanner performed 2,400-s (40 min) list-mode acquisition, and attenuation scans were obtained. Five serial frames were generated. ROI concentration continued to decrease over time and over varying frame durations. There are 2 conclusions. First, scanner does not decay-correct activity to midpoint of first frame or correct for frame duration. Second, in biologic systems, variation in quantitative activity is partly due to inadequate DC or frame duration, which cannot be differentiated from physiologic changes. Therefore, measurement of MBF will not be accurate.
Figure demonstrates relative and quantitative perfusion data of the left anterior descending coronary artery (LAD) territory obtained from a refurbished 2D PET system where decay correction (DC), as part of the default settings within the camera, was performed incorrectly (left column). After recognition of the error, the DC algorithm was corrected and the study reprocessed. 5A and 5B represent rest and stress relative perfusion images, respectively. Both sets of relative images (incorrect and correct DC) are normal and appear nearly identical. 5C and 5D demonstrate inaccurate and accurate DC of rest and stress absolute perfusion in cc/min/g, respectively. With correct DC, rest and stress MBF are ∼30% and ∼55% lower, respectively. 5E and 5F demonstrate the coronary flow capacity (CFC) maps derived by the integration of absolute flow metrics of the incorrect and correct DC datasets, respectively. With incorrect DC, CFC maps suggest physiology consistent with healthy volunteers without risk factors. However, with correct DC, CFC maps are consistent with mild diffuse epicardial disease for which medical therapy is appropriate. Based on CFC maps, treatment would possibly be different based on the absolute perfusion metrics.
THE RATIONALE FOR ALTERNATE DC ALGORITHMS
Most PET cameras are designed and manufactured with a focus on oncologic imaging using low-activity radiotracers (18F and 68Ga) with half-lives significantly longer than the duration of the acquisition. Over the course of a 10- to 15-min oncologic acquisition using these isotopes, loss of activity by radionuclide decay is insignificant such that an alternative DC algorithm could be used (5). Furthermore, in the clinic, SUVs are used instead of absolute quantification of activity. SUV is a ratio of the image-derived radiotracer concentration to the whole-body concentration of injected dose. Provided the calibration time of the injected dose and the start of the acquisition are synchronized, alternative DC algorithms will not impact SUV or nonquantitative data (such as relative perfusion imaging), as whole-body and organ-specific activities are decaying at the same rate and with the same start time.
Therefore, unless the end user tests the scanner specifically for DC for MBF studies, alternative DC algorithms could inadvertently be used, thereby yielding an erroneous MBF. In fact, alternative DC algorithms will pass routine quality control tests when systems are designed for long-lasting radiotracers such as 18F.
However, non-DC datasets could be exported to software that performs DC, as might be used by research laboratories with expertise, but this option is not optimal for primarily clinical services. Finally, in more advanced or research applications, one could apply different DC algorithms based on specific needs since quantitative cardiac imaging is significantly different from oncologic imaging. The half-lives of the approved perfusion tracers are significantly shorter than the duration of the acquisition. Over the course of a myocardial perfusion scan, radiotracer activity decreases about 4-fold for 13N and about 64-fold for 82Rb, hence requiring correct DC.
CONCLUSION
Accurate and precise quantitative myocardial perfusion requires correction for radiotracer decay. DC confirms that changes in activity over the scan duration are due to physiologic changes and not to radiotracer decay, image duration, or framing interval. Testing for correct DC is straightforward and can be performed by onsite technologists with instruments commonly found in a standard PET lab.
DISCLOSURE
Robert Bober is a consultant for Bracco. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
Special thanks are given to Barbara Siede for medical illustrations.
Footnotes
Published online July 30, 2021.
REFERENCES
- Received for publication March 15, 2021.
- Revision received May 5, 2021.












