Abstract
Lymphoscintigraphy plays a vital role in sentinel lymph node (SLN) identification in oncologic breast surgery. The effectiveness of SLN localization and the degree of patient pain were compared between filtered 99mTc-sulfur colloid (99mTc-SC) and 99mTc-tilmanocept. Methods: A retrospective review of patients undergoing lymphoscintigraphy for breast cancer using 99mTc-SC (June 1, 2010, to December 31, 2011) or 99mTc-tilmanocept (June 1, 2013, to January 31, 2014) was performed. SLN appearance time and uptake, SLN pathology, proportion of positive SLNs removed, and pain scores were compared for each radiopharmaceutical using the χ2 test, Fisher exact test, and unequal variance t test, as appropriate. Results: In total, 76 patients, with 86 evaluated axillae, underwent lymphoscintigraphy: 29 with 99mTc-SC and 47 with 99mTc-tilmanocept. The mean SLN appearance time was 11.0 min for 99mTc-SC and 19.3 min for 99mTc-tilmanocept (P = 0.003). There was no difference in the mean transit uptake percentage: 2.2% for 99mTc-SC and 1.9% for 99mTc-tilmanocept (P = 0.55). 99mTc-tilmanocept identified a greater proportion of intraoperative blue nodes than did 99mTc-SC (P = 0.03). There was no significant difference between 99mTc-SC and 99mTc-tilmanocept in the number of SLNs removed, number of patients with positive SLNs, or pain score. Conclusion: 99mTc-SC use in lymphoscintigraphy is an acceptable alternative to 99mTc-tilmanocept for SLN detection in breast cancer, on the basis of the similarity in intraoperative SLN identification and pain scores.
Sentinel lymph node (SLN) surgery continues to play a vital role in staging of breast cancer. When identifying SLNs, a detection method must have adequate sensitivity to detect nodal metastases while maintaining a specificity that will minimize removal of benign lymph nodes. SLNs may be identified via injection of blue dye or lymphoscintigraphy. Two popular radiopharmaceuticals used for lymphoscintigraphy are filtered 99mTc-sulfur colloid (99mTc-SC) and 99mTc-tilmanocept. 99mTc-tilmanocept is composed of a synthetic macromolecule that specifically targets and binds to CD-206 receptors of macrophages found within lymphatic vessels, theoretically targeting SLNs and not migrating to non-SLNs (1). 99mTc-SC is a radiocolloid particle with an average size of 0.3 to 1.0 μm, which is then filtered to a size of less than 0.22 μm before injection to improve lymphatic absorption. The smaller, more uniform particle size is translocated from the injection site into the lymphatic channels, eventually reaching the SLNs draining the injection site; however, unlike 99mTc-tilmanocept, the 99mTc-SC remains unbound and can migrate beyond the sentinel nodes over time (2,3).
Recent studies have shown that the ability of 99mTc-tilmanocept to identify SLNs in breast cancer was superior to that of 99mTc-SC, with less pain on injection (4–17). Two clinical trials performed at the University of California–San Diego showed that 99mTc-tilmanocept exhibited faster injection site clearance and a lower mean number of identified SLNs with a higher concordance than 99mTc-SC, whereas 99mTc-tilmanocept and 99mTc-SC had equivalent SLN uptake (14,16). Similarly, a retrospective study also from the University of California–San Diego showed that 99mTc-tilmanocept patients had fewer nodes removed while having a greater proportion of positive nodes removed among node-positive patients. This study also found that injection with 99mTc-SC independently predicted removal of more than 3 nodes, when adjusted for tumor characteristics (4). Finally, significantly more pain was found to be associated with the 99mTc-SC injection than with the 99mTc-tilmanocept injection (17).
99mTc-SC has been standard at Mayo Clinic–Rochester for SLN biopsy; however, 99mTc-tilmanocept was trialed in a prospective cohort of patients for SLN detection in breast surgery. Both radiopharmaceuticals were evaluated for localization time, transit uptake, ability to intraoperatively localize SLNs, and pain associated with injection. The aim of this study was to determine whether the Mayo Clinic–Rochester experience was similar to previously published reports (4–17).
MATERIALS AND METHODS
After approval by the Institutional Review Board and waiver of the requirement to obtain informed consent, a retrospective review of patients undergoing lymphoscintigraphy for breast surgery using either 99mTc-SC or 99mTc-tilmanocept was performed. Patient data for the 99mTc-SC cohort were retrospectively collected for consecutive patients from June 1, 2010, to December 31, 2011. For the 99mTc-tilmanocept cohort, patient data were collected from June 1, 2013, to January 31, 2014. 99mTc-tilmanocept was trialed at Mayo Clinic–Rochester during this period for use in lymphoscintigraphy for all breast cancer patients; before that time, use of 99mTc-SC had been standard. The 18-mo separation between data collection was to allow for a transition between 99mTc-SC use and 99mTc-tilmanocept use. In total, 76 patients were included in the study, with 86 axillae evaluated. Each axilla was evaluated independently. We excluded patients who were pregnant or breast-feeding, had received prior radiation therapy, had ipsilateral recurrence, or had undergone previous surgery involving the ipsilateral breast tissue. Ten patients underwent bilateral lymphoscintigraphy for bilateral breast surgery.
SLN Identification
An institution-specific standard SLN injection technique was used on all patients. 99mTc-SC patients received 4 intradermal, periareolar injections of 99mTc-SC (0.2-μm filter) in the quadrant of the primary breast tumor. Each syringe contained 3.7–14.8 MBq (0.1–0.4 mCi) of activity in no more than 0.1 mL of saline solution volume. 99mTc-tilmanocept patients received 2 intradermal, periareolar injections of 99mTc-tilmanocept (as manufactured by Navidea Biopharmaceuticals) in the quadrant of the breast tumor. Each syringe was calibrated to contain 18.5–37 MBq (0.5–1.0 mCi) of activity with a total volume of less than 0.4 mL per injection. Immediately after injection, which occurred in the same room as the γ-camera, patients in both groups were imaged for SLN appearance.
Dynamic and static imaging was performed with a γ-camera immediately after injection. With the patients positioned supine and arms above their head, anterior oblique views of the injection site were required for all patients until sentinel node visualization. Any additional imaging was acquired as needed. If patients received bilateral injections, static anterior views were acquired in addition to the anterior oblique views required for each side. A 57Co sheet source was used as a transmission source.
Localization time was defined as the elapsed time from radiotracer administration to sentinel node visualization as indicated on patient images by the imaging technologist. A sentinel node was confirmed by the reading nuclear medicine physician or radiologist and was annotated on final patient images. Manual regions of interest (ROIs) were drawn around the injection site and sentinel nodes, as identified by the physician, on anterior oblique images to yield count information. These values were then used for mathematic manipulation to determine the transit uptake percentage using the equation below: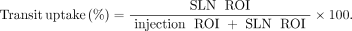
During surgery, SLNs were identified using radionuclide activity via a γ-probe with or without the addition of methylene blue dye. Excised nodes were submitted for pathologic examination. Pathology reports were reviewed for reported blue nodes, number of SLNs removed, and positive SLNs. Patients who did not undergo SLN surgery, because they were undergoing breast surgery for risk reduction or atypia, were excluded from intraoperative SLN identification analyses. The excluded axillae included 1 (from 1 patient) in the 99mTc-SC group and 18 (from 15 patients) in the 99mTc-tilmanocept group.
Pain Associated with Intradermal Injection
Only patients who received a topical eutectic mixture of local anesthetic cream before injection were included in the pain analysis, which consisted of 22 women in the 99mTc-SC group and 47 in the 99mTc-tilmanocept group. For all patients, the anesthetic cream was applied to the skin and covered with an adhesive patch around the areola in the quadrant of the tumor 30 min before the injections. Intradermal periareolar injections were performed with a 25-gauge needle by the nuclear medicine radiologist using sterile technique. 99mTc-SC was given with 4 injections per breast, each containing 3.7–14.8 MBq (0.1–0.4 mCi) in no more than 0.1 mL of saline solution volume. 99mTc-tilmanocept was given with 2 injections per breast, each containing 18.5–37 MBq (0.5–1.0 mCi) in less than 0.4 mL of volume. Patients were asked to give a pain score immediately after the injections using a linear pain scale from 0 to 10 (0, no pain; 10, unbearable pain).
Statistical Analysis
Data were compared between the 99mTc-SC and 99mTc-tilmanocept groups using the χ2 test, Fisher exact test, and unequal variance t test, as appropriate. All analyses were completed using JMP statistical software, version 10.0. The α-level for statistical significance was set at 0.05.
RESULTS
SLN Identification
In total, 76 patients, with 86 evaluated axillae, underwent lymphoscintigraphy: 29 with 99mTc-SC (29 axillae) and 47 with 99mTc-tilmanocept (57 axillae). The average patient age was 57.0 y in the 99mTc-SC group and 59.5 y in the 99mTc-tilmanocept group (P = 0.22) (Table 1). In the 99mTc-SC group, more patients underwent lumpectomy than mastectomy (18/29 [62.1%] vs. 11/29 [37.9%]), whereas more patients underwent mastectomy than lumpectomy in the 99mTc-tilmanocept group (22/47 [46.8%] vs. 25/47 [53.2%]); this difference was not statistically significant (P = 0.24) (Table 1). One patient in the 99mTc-tilmanocept group did not undergo concurrent breast surgery, because no breast lesion was seen on preoperative imaging and the patient declined a breast operation.
Nuclear Medicine Data
Localization Time
The average localization time for the 99mTc-SC group was 11.0 min ± 7.4, versus 19.3 min ± 18.1 for the 99mTc-tilmanocept group (P = 0.003). Of the 29 axillae in the 99mTc-SC cohort, 25 (86.2%) had a visible SLN within 12 min from the time of injection, 1 (3.5%) within 18 min, and 3 (10.3%) within 30 min. For the 57 axillae in the 99mTc-tilmanocept cohort, 37 (64.9%) had a verified SLN appearance within 12 min, and an additional 9 (80.7% total) had a verified SLN appearance when the time was extended to 18 min. Five (8.8%) had a localization time of 30 min, and 6 (10.5%) had a localization time of more than 30 min. Total nodes visualized were 31 (average of 1.1 per patient) for the 99mTc-SC group and 77 (average of 1.3 per patient) for the 99mTc-tilmanocept group (P = 0.02).
The average transit uptake for the 99mTc-SC group was 2.2% ± 2.4%, versus 1.9% ± 2.7% for the 99mTc-tilmanocept group (P = 0.55).
Pain Associated with Intradermal Injection
The 99mTc-SC group had a higher mean pain score, at 4.2 ± 2.3, versus 3.3 ± 2.6 for the 99mTc-tilmanocept group; however, this difference was not statistically significant (P = 0.16) (Fig. 1). Additionally, the pain score was 5 or higher in 44.4% (4/9) of the 99mTc-SC patients but in only 20.4% (10/49) of the 99mTc-tilmanocept patients.
Average localization time for 99mTc-tilmanocept vs. 99mTc-SC.
SLN Surgery
Of the 29 99mTc-SC patients, 28 underwent SLN surgery. All had SLNs identified using a γ-probe. The average number of SLNs removed was 2.6 ± 1.6 (range, 1–9). Six patients had positive nodes, ranging in number from 1 to 2. Of the 57 breasts injected with 99mTc-tilmanocept, 39 underwent SLN surgery. The average number of SLNs removed was 2.4 ± 1.6 (range, 1–8). Five patients had positive SLNs, all with 1 positive node. There was no statistical difference between groups in the average number of SLNs removed, the number of positive nodes, or the proportion of positive nodes excised (P = 0.66, 0.89, and 0.72, respectively) (Table 2).
SLN Data
Of all patients injected with methylene blue dye who underwent SLN surgery, 15 of 27 (55.5%) 99mTc-SC patients had blue SLNs identified, 2 of which were positive for metastasis. In the 99mTc-tilmanocept group, 22 of 30 (73.3%) patients had blue nodes, 3 of which were positive for metastasis. The proportion of blue nodes identified as SLNs was greater for the 99mTc-tilmanocept group than for the 99mTc-SC group (P = 0.03) (Table 2).
DISCUSSION
SLN Identification
The localization time for the 99mTc-SC group was 11 min, versus 19 min for the 99mTc-tilmanocept group, suggesting that lymphatic transit time may be quicker for 99mTc-SC than for 99mTc-tilmanocept with standard use. Additionally, fewer SLNs were identified with 99mTc-SC than with 99mTc-tilmanocept, although the difference was not clinically significant. There was no statistical difference in the other evaluated variables, including transit uptake, intraoperative SLN identification, or percentage of positive node identification.
The faster localization time for 99mTc-SC may be beneficial for institutions that inject intraoperatively, with the surgeon having to wait for the radiopharmaceutical activity to be present in the axilla before proceeding. Additionally, one perceived benefit of 99mTc-tilmanocept over 99mTc-SC is that the large size and macrophage-specific receptor binding of 99mTc-tilmanocept prevent it from traveling to non-SLNs (1). Our data showed that fewer SLNs were identified with 99mTc-SC, whereas the percentage of positive SLNs identified in node-positive patients remained statistically equivalent. This finding suggests that the smaller size of 99mTc-SC and its absence of a specific binding target do not limit its ability to identify SLNs intraoperatively and that 99mTc-SC remains at least equivalent to 99mTc-tilmanocept for this purpose.
Previous studies, by Wallace et al., that evaluated the use of 99mTc-SC versus 99mTc-tilmanocept in SLN identification showed that SLNs removed from patients in the 99mTc-tilmanocept group were more concordant with blue dye and that 99mTc-tilmanocept cleared more quickly from the injection site (14,16). Our study did not evaluate clearance time; however, the 99mTc-tilmanocept group had a greater number of SLNs identified by imaging and an equivalent number identified intraoperatively. Our study did agree with previous studies in identifying a greater proportion of blue SLNs in the 99mTc-tilmanocept group. Primary SLN uptake in our study was greater than that reported by Wallace et al. for both groups, but neither study found a statistical difference between the 2 radiopharmaceuticals (16). Reasons for differences between our study and previously published prospective studies may include the larger sample size and the retrospective design of our study.
Baker et al. also performed a retrospective review of 84 99mTc-tilmanocept and 115 99mTc-SC patients (4). Their study showed that fewer SLNs were identified in the 99mTc-tilmanocept group than in the 99mTc-SC group (4). Additionally, they found that both groups had a similar proportion of metastatic lymph node–positive patients; however, the 99mTc-tilmanocept group identified a greater number of positive nodes among the node-positive patients (4). Collected data in our study suggest that a similar number of SLNs is identified in the 2 groups, with an equal proportion of positive nodes identified in each group. Reasons for the difference between our findings and those of Baker et al. likely include the small sample size and small proportion of patients with positive lymph nodes in both groups in the previously published study.
Pain Associated with Intradermal Injection
Our study found no significant difference in pain between the 99mTc-SC and 99mTc-tilmanocept groups. A prior randomized controlled trial found more pain associated with 99mTc-SC than with 99mTc-tilmanocept within the first 3 min after injection (17). The topical eutectic mixture of local anesthetic cream was applied preoperatively to all patients in our study, which may have helped to eliminate differences in the injection-associated pain; however, a previous study from Mayo Clinic–Rochester showed that topical anesthetic cream did not help with injection pain (18). Today, these findings may be of limited clinical importance as all patients now receive intradermal lidocaine at the injection sites at Mayo Clinic–Rochester, which has been shown to improve patient tolerance to the procedure (19).
Limitations
Limitations to this study include its retrospective design and modest sample size. Additionally, although patients were injected in the same room as the γ-camera, they were not injected directly beneath the camera with immediate dynamic imaging to ensure the most accurate measurement of transit time. Thus, the time to perform the injections and patient transport time from injection to imaging may have made the times to visualization appear longer than the actual transit times. Also, in the pain analysis, patients injected with 99mTc-SC had 4 injections, whereas 99mTc-tilmanocept only had 2 injections. Multiple injection sites were chosen to ensure injection on each side of a tumor or scar to see all possible drainage patterns. Even with the difference in the number of injections between 99mTc-tilmanocept and 99mTc-SC, there was only a slightly lower mean pain score for 99mTc-tilmanocept and no statistical difference was found. Finally, 99mTc-SC and 99mTc-tilmanocept could not be directly compared in the same patient. To do so, one would need to inject a patient with one of the agents, wait for radioactivity to decrease to zero, inject the patient with the other agent, and then proceed to surgery. Not only is this unreasonable from a patient standpoint, but no pathologic comparison data would be available, as only one of the injections would be followed by operative intervention.
CONCLUSION
Comparison of 99mTc-SC and 99mTc-tilmanocept for lymphoscintigraphy to detect SLNs in breast cancer showed that the former continues to be an acceptable alternative to the latter. This conclusion is based on their similarity in intraoperative SLN identification and in patient-perceived pain.
DISCLOSURE
The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Brittany Murphy. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Apr. 24, 2019.
REFERENCES
- Received for publication December 23, 2018.
- Accepted for publication March 1, 2019.








