Abstract
Quick methods are functional in clinical practice to ensure the fastest availability of radiopharmaceuticals. For this purpose, we investigated the radiochemical purity of the widely used 99mTc-hydroxymethylene diphosphonate, 99mTc-hexamethylpropyleneamine oxime, and 99mTc-tetrofosmin by reducing time as compared with the manufacturer's method. Methods: We applied a miniaturized chromatographic method with a reduced strip development from 18 cm to 9 cm for all 3 radiopharmaceuticals. The specific support medium and solvent system of the manufacturer's methods was kept unchanged for 99mTc-hydroxymethylene diphosphonate and 99mTc-tetrofosmin, whereas for 99mTc-hexamethylpropyleneamine oxime the instant thin-layer chromatography (ITLC) polysilicic gel (silicic acid [SA]) was replaced with a monosilicic gel (silicic gel [SG]) in the chromatographic system that uses methyl ethyl ketone as solvent. The method was applied and compared with the routine ITLC insert method in a total of 30 batches for each radiopharmaceutical. The precision of repeated tests was determined by comparison with the results of 10 replications on the same batch. Small volumes of concentrated 99mTcO4−, and 99mTc-albumin nanocolloid were used to produce potential radiochemical impurities. Correlation between the quick methods and the insert methods was analyzed using a nonparametric 2-tailed test and a 2 × 2 contingency table with the associated Fisher exact test to evaluate sensitivity and specificity. A receiver-operating-characteristic analysis was performed to evaluate the best cutoff. Results: The percentage radiochemical purity of the quick methods agreed with the standard chromatography procedures. We found that 99mTcO4 and colloidal impurities are not the only common radiochemical impurities with 99mTc-tetrofosmin, and shortening of the ITLC strip with respect to the manufacturer's method will worsen system resolution and may produce inaccuracy. Conclusion: The miniaturized methods we described represent a fast and reliable alternative for 99mTc-exametazime and 99mTc-oxidronate quality control, with the upper cutoff for acceptable radiochemical purity values being 84% and 95%, respectively. For 99mTc-tetrofosmin radiochemical purity testing, a longer strip as described in the standard method is warranted.
Testing of radiochemical purity is crucial for quality control assurance of radiopharmaceuticals in the daily routine of any nuclear medicine department. The procedures recommended by the manufacturer’s package insert or by monographs in the European Pharmacopeia (1–3) for 99mTc-hydroxymethylene diphosphonate (HMDP), 99mTc-hexamethylpropyleneamine oxime (HMPAO), and 99mTc-tetrofosmin radiochemical purity testing are time consuming both for setting and development time. This makes the adequate, timely use of these radiopharmaceuticals problematic for some specific applications (such as radiolabeling of autologous leukocytes, which is optimal with freshly prepared 99mTc-HMPAO). In addition, it also conflicts with their useful shelf-life.
Over 35 y ago, Zimmer and Pavel (4) validated quick miniaturized chromatographic systems for radiopharmaceuticals that were at that time widely used in nuclear medicine, including 99mTc-labeled sulfur colloid, macroaggregated albumin, stannous chloride, phytate, dimercaptosuccinic acid, diethylene triamine pentaacetic acid, pyrophosphate, diphosphonate, methylene diphosphonate, polyphosphate, and glucoheptonate. However, many new radiopharmaceuticals have subsequently been introduced into clinical practice, thus requiring validation of the corresponding miniaturized radiochemical purity tests (5–7).
In the case of 99mTc-HMDP, the use of a modified Zimmer and Pavel technique performs poorly, as the peak of 99mTc-HMDP occurs earlier than the expected relative front (Rf) and thus overlaps with the peak of hydrolyzed reduced technetium (HRTc) (5).
The evolution of radiochemical purity testing for 99mTc-HMPAO has had some interesting twists. A miniaturized chromatographic radiochemical purity test was recommended in the original package insert of the commercial 99mTc-HMPAO labeling kit (revised in February 2006); this method was replaced in January 2013 with a nonminiaturized method (8), most probably because of the difficulty in obtaining the desired separation of radiolabeled species using the traditional silica gel strips. Even more recently (in June 2013), the manufacturers replaced silica gel with silicic acid (8).
On the other hand, the rapid radiochemical purity testing methods described so far for quality control of 99mTc-tetrofosmin either have low sensitivity for unacceptable radiochemical purity values (9) or have been validated only with high-purity batches (10).
These considerations prompted us to modify the chromatographic procedure for radiochemical purity testing of 99mTc-HMDP, 99mTc-HMPAO, and 99mTc-tetrofosmin recommended by the manufacturers, with the aim of making them less time consuming. We report here the results of a series of tests performed for validation of such modified techniques.
MATERIALS AND METHODS
Radiopharmaceuticals
Thirty commercial kits of 99mTc-HMDP, 99mTc-HMPAO, and 99mTc-tetrofosmin were reconstituted according to the package insert instructions. Since these radiopharmaceuticals generally do not present significant impurities, to demonstrate the ability of the system to detect radiochemical purity values below the accepted limit we added 99mTcO4− and 99mTc-albumin nanocolloids. Therefore, in 10 preparations for each radiopharmaceutical we incorporated high concentrations and small volumes of 99mTc-sodium pertechnetate or 99mTc-albumin nanocolloids. The latter was chosen as a surrogate for HRTc (insoluble 99mTc-dioxide or 99mTc-tin colloid) or for other hydrophilic impurities that stay at the origin in most instant thin-layer chromatography (ITLC) systems (11).
Miniaturized Methods
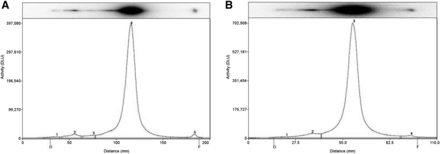
The manufacturer’s radiochemical purity testing methods were modified as follows: we reduced the size of the ITLC strips, which was set at 1 × 10.5 cm for all 3 radiopharmaceuticals (vs. 2 × 20 cm in the standard methods) and we reduced migration in the ITLC strip from the 15 cm of the standard methods to 7.5 cm (Fig. 1).
Chromatography diagrams of alternative (A) and standard (B) methods for radiochemical purity testing of radiopharmaceuticals.
Each strip was marked to ensure accurate migration levels: 1.5 cm as the origin line and 9 cm as the front line. Marking-pen lines were placed accordingly, to ensure that no overlap occurred between the deposited radiopharmaceuticals and the marker. The solid phase was maintained as (Whatman) paper and salicylic acid gel (ITLC-SA) for 99mTc-HMDP and 99mTc-tetrofosmin, respectively, whereas in the case of 99mTc-HMPAO, inactivated silica gel ITLC-SG was used instead of ITLC-SA as the solid phase, with MeOH as the solvent. We kept unchanged all other components of the solvent system with respect to the corresponding manufacturer’s methods (Table 1).
Rf Ranges for Peaks of Most Common 99mTc Components in Radiopharmaceutical Preparations
For all the procedures, we used adequately calibrated pipettes to control the size of the spotted samples and to measure solvents; polypropylene blood withdrawal tubes were used as ascending chromatographic chambers.
Radiochemical purity of each radiopharmaceutical batch obtained as described above was evaluated under its useful shelf-life using both the manufacturer’s and the miniaturized methods. To obtain 10 low-purity batches of 99mTc-HMPAO, radiochemical purity testing was performed 30–60 min after reconstitution.
A phosphor imager (Cyclone Plus; Perkin Elmer) was used for the identification and quantification of radioactivity distribution along the chromatographic strips. Resolution greater than 1.5 was accepted to minimize integration errors possibly due to manual integration of the chromatographic peaks. The Rf range was kept unchanged for 99mTc-HMPAO and 99mTc-HMDP, whereas it was set at 0.3–0.9 for 99mTc-tetrofosmin (vs. 0.25–0.8 as recommended in the manufacturer’s method) (Table 1).
Statistical Analysis
Correlations between the quick methods and the standard methods were evaluated using a nonparametric 2-tailed test and a 2 × 2 contingency table, whereas the associated Fisher exact test was used to assess sensitivity and specificity. An α-value of 0.05 was used to determine the validity of the new procedures. Precision of the modified methods was assessed by repeating 10 times the radiochemical purity test using samples from a single preparation for each radiopharmaceutical. Finally, receiver-operating-characteristic analysis was performed to identify the best cutoff for radiochemical purity testing.
RESULTS
99mTc-HMDP
The modified method shortened considerably the time required for radiochemical purity testing of 99mTc-HMDP to complete the quality control procedure, mainly by reducing the migration time from 50 min for the standard method to 5 min only for the miniaturized strips.
In all 30 samples investigated with both methods using saline solution as the solvent, 99mTc-HMDP moved at the solvent front as a long smear rather than as a single discrete spot. This migration pattern prevented exact detection of HRTc impurities, because about 2% of the final 99mTc-HMDP compound was found in the expected Rf range of HRTc impurity. In contrast, 98% of the total 99mTc-HMDP activity was in the expected Rf range for the pure radiopharmaceutical (extending throughout 0.4–1). When 99mTc-pertechnetate was added and MeOH/H2O was used as the solvent, it produced a sharp peak separated at baseline with an Rf value of 0.8–1, in both the standard and the miniaturized methods (Table 1).
Figure 2 shows the correlation between the radiochemical purity values obtained with the 2 methods (R = 0.94, P < 0.0001). With the miniaturized method, sensitivity for the detection of unacceptable radiochemical purity values (<95% purity) and specificity for acceptable radiochemical purity values (>95% purity) were both 100% (P < 0.0001 by the Fisher exact test), with accuracy ranging from 99% to 104%. Interassay variability was 0.4% for the manufacturer’s method and 0.8% for the modified method.
Radiochemical purity of 99mTc-HMPAO (left), 99mTc-HMDP (middle), and 99mTc-tetrofosmin (right), obtained by standard and miniaturized strips in commercial reconstituted samples. Correlation coefficients were, respectively, 0.97, 0.93, and 0.86 (with P = 0.0013, P < 0.0001, and P < 0.0001, respectively).
99mTc-HMPAO
Radiochemical purity testing of 99mTc-HMPAO using the manufacturer’s instructions took approximately 30 min, because of the long chromatographic run and set-up procedures, whereas the miniaturized method with inactivated ITLC-SA/ITLC-SG strips was completed within approximately 10 min.
In the standard method based on the use of ITLC-SA and saline as the solvent, the lipophilic 99mTc-HMPAO complex, the secondary 99mTc-HMPAO complex, and HRTc migrated with a unique peak ranging from Rf 0 to 0.2. We found no difference in 99mTc-pertechnetate resolution when we used the miniaturized method.
Nevertheless, in the methyl ethyl ketone standard system, which quantifies the secondary hydrophilic 99mTc-HMPAO complex and HRTc, ITLC-SG provided better resolution of those radiochemical species than ITLC-SA.
Figure 2 shows the close correlation between radiochemical purity values obtained with the miniaturized method and with the manufacturer’s method, respectively (R = 0.97; P < 0.0001).
Using the modified method, a 2 × 2 contingency table showed 92.8% sensitivity for the detection of unacceptable radiochemical purity values (<80% purity) and 100% specificity for acceptable radiochemical purity values (>80% purity). The associated Fisher exact test yielded a P value of less than 0.0001. With the very low-purity batches, the relative increase of the peak size for impurity with resolution below 1.0 generated unacceptable errors that prevented the calculation of accuracy. Interassay variability was under 1% for both methods. Receiver-operating-characteristic analysis showed that the best cutoff for the quick miniaturized method is 84% of radiochemical purity (100% sensitivity and 100% specificity, P < 0.0001).
99mTc-Tetrofosmin
The quick, miniaturized radiochemical purity method for 99mTc-tetrofosmin requires only 5 min to be completed, thus comparing very favorably with the standard method (20 min).
We identified several critical technical issues of radiochemical purity testing for 99mTc-tetrofosmin, including accuracy when preparing the sample volume, use of a freshly prepared solvent solution with strict control of the acetone-to-dichloromethane ratio, and meticulous attention to the solvent front, which should not migrate beyond the front line.
With the manufacturer’s method, free 99mTc-pertechnetate runs to the top of the strip (Rf = 0.8–1), 99mTc-tetrofosmin migrates to the center of the strip (Rf = 0.5–0.6), and colloidal impurities remain at the origin, along with 2 reduction impurities (Rf = 0.1–0.2) (Fig. 3).
ITLC-SA with acetone:dichloromethane (85:65) of batch of 99mTc-tetrofosmin: (A) Manufacturer's method: radiochemical purity, 94.4%. (B) Miniaturized method: radiochemical purity, 93.7%. Impurities are HRTc (peak 1), unknown impurities (peaks 2 and 3), 99mTc-tetrofosmin (peak 4), and 99mTcO4− (peak 5), accounting for 2% of total radioactivity.
When following the labeling instructions, we never found free 99mTc-pertechnetate in any of the final preparations, except those where 99mTc-pertechnetate was added on purpose.
With the quick, modified method, the system’s resolution worsened because the peak of 99mTc-tetrofosmin migrates more closely to the front line (Rf = 0.6–0.7), thus partly overlapping with the 99mTc-pertechnetate peak. Nevertheless, despite the poorer resolution, the presence of a 2% impurity of 99mTc-pertechnetate (or even more, as evaluated by the reference standard method) can readily be displayed with the quick method. Colloidal impurities did not migrate from the origin (Rf = 0), thus being well separated from the 99mTc-tetrofosmin peak. The peaks of 2 additional unknown hydrophilic complex impurities at the bottom portion of the strips were found to overlap with each other and with the 99mTc-tetrofosmin peak.
Figure 2 shows the correlation between the radiochemical purity values obtained with the 2 methods (R = 0.85, P < 0.0001). Sensitivity of the quick miniaturized method for the detection of unacceptable radiochemical purity values (<90% purity) was 96%, whereas specificity for acceptable radiochemical purity values (>90% purity) was 100% (P < 0.0001 by the Fisher exact test). Accuracy ranged from 98% to 102%. Interassay variability was 0.4% for the manufacturer’s method and 0.7% for the modified method. Receiver-operating-characteristic analysis yielded a 99% overall ability of the test to discriminate between conformity and nonconformity (P < 0.0001), with 100% sensitivity and specificity with a 92% cutoff for radiochemical purity.
DISCUSSION
The miniaturized methods presented here include several modifications introduced with the purpose of speeding up the standard methods for radiochemical purity testing of 3 widely used radiopharmaceuticals: 99mTc-HMDP, 99mTc-HMPAO, and 99mTc-tetrofosmin. A critical feature of the miniaturized methods consists of reducing the size of the ITLC strips, with consequent shortening of the migration time.
The main advantage of the quick method over the standard method for 99mTc-HMDP is the considerable shortening of the time required to complete testing. In fact, reduction of solvent migration from 15 cm (standard method) to 7.5 cm (miniaturized method) translates into shortening from 50 min to 5 min.
The miniaturized method for 99mTc-tetrofosmin shows an Rf of 0.3–0.8 as previously reported by McKay et al. (6) in a similar miniaturized system, which however has been validated only with high-purity batches (>90%). Addition of free 99mTc-pertechnetate worsened resolution of the miniaturized system, due to partial overlap between the 99mTc-tetrofosmin and the free 99mTc-pertechnetate peaks. However, it should be noted that free 99mTc-perthecnetate was never found in any of the standard preparations. In addition, whereas the novel quick method clearly detected HRTc impurity in the bottom portion of the strip, it did not discriminate the 2 reduction impurity species described in the literature as either a Tc(IV) or a Tc(III) product (12). Therefore, we can argue that miniaturized methods might produce inaccurate results when radiochemical purity values lie near the cutoff line.
Although no changes in the solid phase or in the solvent systems were made for 99mTc-HMDP and 99mTc-tetrofosmin, in the radiochemical purity testing for 99mTc-HMPAO (where methyl ethyl ketone is used as the solvent) the solid phase was changed from monosilicic ITLC-SA to ITLC-SG. In this regard, radiochemical purity testing for 99mTc-HMPAO is especially problematic due to the short time window between reconstitution and injection of this radiopharmaceutical into patients. Therefore, we resorted to use of a miniaturized system that take approximately 5 min to develop, use of blood collection tubes as developing chambers that require 5 min to saturate with solvent, and use of ITLC-SG instead of monosilicic gel ITLC-SA, which performs better with methyl ethyl ketone as solvent.
CONCLUSION
The quick, miniaturized method here described for quality control of 99mTc-HMDP and 99mTc-HMPAO with reproducible radiochemical purity values represents a valid alternative to the standard methods. In contrast, in the case of 99mTc-tetrofosmin radiochemical purity testing, the use of a longer strip as described in the standard method is strongly recommended.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jul. 13, 2017.
REFERENCES
- Received for publication January 20, 2015.
- Accepted for publication June 20, 2017.