Abstract
A major disadvantage of 18F-FDG PET involves poor detection of small lesions and lesions with low metabolism, caused by limited spatial resolution and relatively large image voxel size. As spatial resolution and sensitivity are better in new PET systems, it is expected that small-lesion detection could be improved using smaller voxels. The aim of this study was to test this hypothesis using a state-of-the-art time-of-flight PET/CT device. Methods: 18F-FDG PET scans of 2 image-quality phantoms (sphere sizes, 4–37 mm) and 39 consecutive patients with lung cancer were analyzed on a time-of-flight PET/CT system. Images were iteratively reconstructed with standard 4 × 4 × 4 mm voxels and smaller 2 × 2 × 2 mm voxels. For the phantom study, we determined contrast-recovery coefficients and signal-to-noise ratios (SNRs). For the patient study, 18F-FDG PET–positive lesions in the chest and upper abdomen with a volume less than 3.0 mL (diameter, <18 mm) were included. Lesion mean and maximum standardized uptake values (SUVmean and SUVmax, respectively) were determined in both image sets. SNRs were determined by comparing SUVmax and SUVmean with background noise levels. A subanalysis was performed for lesions less than 0.75 mL (diameter, <11 mm). For qualitative analysis of patient data, 3 experienced nuclear medicine physicians gave their preference after visual side-by-side analysis. Results: For phantom spheres 13 mm or less, we found higher contrast-recovery coefficients and SNRs using small-voxel reconstructions. For 66 included 18F-FDG PET–positive lesions, the average increase in SUVmean and SUVmax using the small-voxel images was 17% and 32%, respectively (P < 0.01). For lesions less than 0.75 mL (21 in total), the average increase was 21% and 44%, respectively. Moreover, averaged over all lesions, the mean and maximum SNR increased by 20% and 27%, respectively (P < 0.01). For lesions less than 0.75 mL, these values increased up to 23% and 46%, respectively. The physicians preferred the small-voxel reconstructions in 76% of cases. Conclusion: Supported by a phantom study, there was a visual preference toward 18F-FDG PET images reconstructed with 2 × 2 × 2 mm voxels and a profound increase in standardized uptake value and SNR for small lesions. Hence, it is expected that small-lesion detection improves using small-voxel reconstructions.
Whole-body 18F-FDG PET integrated with CT is widely used for primary tumor analysis and mediastinal lymph node staging in patients with non–small cell and small cell lung cancer (1,2). For these patients, accurate lymph node staging is of high importance because both prognosis and therapeutic management depend on the tumor stage (3). A major disadvantage of 18F-FDG PET involves poor detection of small lesions and lesions with low metabolism. With a sensitivity of 76% and specificity of 88% (4), PET/CT is less sensitive for nodal involvement of small nodes (diameter, <10 mm) (3,5). This poor detection is caused by limited spatial resolution and sensitivity (3,6). To improve work flow and patient comfort, relatively large image voxel sizes are generally preferred in clinical practice to effectively obtain more counts per voxel and subsequently a reduction in image noise (7). However, the low spatial resolution introduces the partial-volume effect, which affects images both visually and quantitatively because small lesions may appear larger and their 18F-FDG uptake may seem lower than is actually the case (8). Consequently, the detection of small lesions is limited by the partial-volume effect.
In the literature, several ways to improve lesion detectability on PET/CT are described. With the introduction of scintillators such as lutetium orthosilicate and lutetium yttrium orthosilicate, time-of-flight (TOF) PET became the new standard technology for PET manufacturers. With the implementation of TOF PET, the increased signal-to-noise ratio (SNR) appeared to improve the detectability of small lesions (9–12). Furthermore, the detection of small lesions can be improved by changing reconstruction settings, for example, by optimizing the number of iterations and subsets and by modeling of the point-spread function (13–15).
In current practice, the image voxel size for whole-body 18F-FDG PET scans is typically around 4 × 4 × 4 mm (7,16). Conti (7) suggested that in combination with new high sensitive TOF PET/CT cameras, the use of reconstructions with smaller voxels might further improve the detection of small lesions. However, it was not assessed before to which extent small lesion detectability is influenced by the reconstruction voxel size. Therefore, the aim of this study was to determine the impact of a small-voxel reconstruction on the detectability of small lesions using a state-of-the-art TOF PET/CT device.
MATERIALS AND METHODS
To study the impact of small-voxel reconstructions on lesion detectability using a state-of-the-art TOF PET/CT device, we first performed a phantom study and subsequently a patient study.
Phantom Study
We analyzed 2 image-quality phantoms consisting of spheres with varying sizes. The National Electrical Manufacturers Association (NEMA) International Electrotechnical Commission (IEC) Body Phantom Set (model PET/IEC-BODY-P) image-quality phantom (NEMA phantom) has an interior length of 18 cm and contains 6 fillable spheres with 10-, 13-, 17-, 22-, 28-, and 37-mm inner diameters. The large background compartment (with a volume of 9,300 mL) and the 6 spheres were filled with a solution of 18F-FDG, 2.0 and 19.9 kBq/mL, respectively, resulting in a sphere-to-background ratio of 10.0. The sphere-to-background ratio was consistent with the guidelines of the European Association of Nuclear Medicine (EANM) (17). The cylinder-shaped Micro Hollow Sphere Phantom (model ECT/HS/MMP) (microphantom) has an inside diameter of 40 mm, inside height of 82 mm, and volume of 103 mL. It contains 4 fillable spheres with 4-, 5-, 6-, and 8-mm inner diameters. The background compartment and 4 spheres were filled with a solution of 18F-FDG, 1.9 and 17.4 kBq/mL, respectively, resulting in a sphere-to-background ratio of 9.4.
Patient Study
Thirty-nine consecutive patients with lung cancer, who underwent 18F-FDG PET/CT, were analyzed. Patients signed a written informed consent form, and this study was approved by the local ethical committee of our institution. Patients fasted for at least 6 h before scanning. Before intravenous injection of 18F-FDG, blood glucose levels were measured to ensure a value below 15 mmol/L. A dedicated dose protocol, depending quadratically on patients’ body weight (18), was implemented using the formula A × t = 3.8 × m2. In this formula, A is the 18F-FDG dose to administer in MBq, t the time per bed position in seconds (s), and m the body weight in kilograms (kg). 18F-FDG dose and acquisition time were consistent with EANM guidelines for tumor PET imaging (17).
Data Acquisition
All PET/CT scans were acquired with the Ingenuity TF PET/CT scanner (Philips Healthcare). This fully 3-dimensional (3D) TOF scanner is combined with a 128-slice CT scanner. The PET system contains 28,336 lutetium yttrium orthosilicate crystals (size, 4 × 4 × 22 mm) divided across 44 detector rings. Regarding TOF performance, the timing resolution of the PET scanner is 495 picoseconds, with a TOF localization accuracy of 7.43 cm. The scan duration for the NEMA phantom was 10 min per bed position, as proposed in the EANM guidelines (17). Also for the microphantom, the duration of the PET scan was 10 min. Acquisition times for the patient studies were, respectively, 1 and 2 min per bed position for patients with a body weight 80 kg or less and more than 80 kg. The administered 18F-FDG dose ranged from 185 to 500 MBq. The PET scan was followed by a CT scan, used for attenuation correction.
Data Reconstruction
PET data were reconstructed using a default 3D ordered-subset iterative TOF reconstruction technique. Images were reconstructed in 2 types of matrices: 144 × 144 matrices with a voxel size of 4 × 4 × 4 mm (standard-voxel reconstruction) and 288 × 288 matrices with a voxel size of 2 × 2 × 2 mm (small-voxel reconstruction). To compensate for detector blurring, a blob-based reconstruction was applied. These spheric symmetric image elements (blobs), originating from Kaiser–Bessel basis functions (19,20), are used instead of voxels for image representation. In a blob reconstruction, there is blob overlap, enabling better contrast-to-noise performance without the need for postfiltering (20). We used 2 types of blobs in this study. For the standard-voxel reconstruction, the blob had a 2.5-mm radius, with a blob shape parameter of 8.4 mm. The blob radius and shape parameter for the small-voxel reconstruction were 2.8 and 6.4 mm, respectively. Furthermore, the relaxation parameters for the standard- and small-voxel reconstruction were 1.0 and 0.5, respectively. For both types of voxel reconstructions, 3 iterations and 43 subsets were applied. All reconstruction parameters were default settings recommended by the manufacturer.
Data Analysis
Phantom Study
Quantitative measurements were performed on a dedicated workstation (IntelliSpace Portal 6; Philips Healthcare). For each sphere, we determined the mean and maximum 18F-FDG concentration (kBq/mL) in both standard- and small-voxel reconstructed image sets. The mean 18F-FDG concentration was calculated from a 3D isocontour created at 50% of the maximum pixel value. Furthermore, background measurements were performed in a circle-based region of interest (ROI) of approximately 2,000 mm2 (NEMA phantom) and 400 mm2 (microphantom) localized in a homogeneous region in a background part of the phantom. For the NEMA phantom, we performed background measurements on the most central axial slice, at least 20 mm away from both the phantom edge and the phantom spheres to prevent influence of the partial-volume effect. For the microphantom, we performed the background measurements in an axial slice 20 mm below the spheres. The mean 18F-FDG concentration and SD in the ROI were determined. Using Equation 1, we calculated the noise in the phantom background compartment.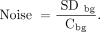 Eq. 1In this formula, Cbg and SDbg are, respectively, the mean and SD of the 18F-FDG concentration as measured in the background of the reconstructed images. For each sphere, we determined the mean and maximum contrast recovery coefficients (CRCmean and CRCmax, respectively) using Equation 2.
Eq. 1In this formula, Cbg and SDbg are, respectively, the mean and SD of the 18F-FDG concentration as measured in the background of the reconstructed images. For each sphere, we determined the mean and maximum contrast recovery coefficients (CRCmean and CRCmax, respectively) using Equation 2.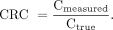 Eq. 2CRC is defined as the ratio between the measured (maximum or mean) 18F-FDG concentration in the images (Cmeasured) and the true 18F-FDG concentration in the sphere (Ctrue). Cmeasured and Ctrue were both adapted for the background, to distinguish the uptake in the (small) spheres from background uptake. Hence, for the NEMA phantom spheres Ctrue was 17.9 kBq/mL, and for the microphantom spheres Ctrue was 15.5 kBq/mL. Furthermore, we calculated the mean and maximum SNR (SNRmean and SNRmax, respectively) using Equation 3.
Eq. 2CRC is defined as the ratio between the measured (maximum or mean) 18F-FDG concentration in the images (Cmeasured) and the true 18F-FDG concentration in the sphere (Ctrue). Cmeasured and Ctrue were both adapted for the background, to distinguish the uptake in the (small) spheres from background uptake. Hence, for the NEMA phantom spheres Ctrue was 17.9 kBq/mL, and for the microphantom spheres Ctrue was 15.5 kBq/mL. Furthermore, we calculated the mean and maximum SNR (SNRmean and SNRmax, respectively) using Equation 3. Eq. 3In this equation, SDbg is the SD of the 18F-FDG concentration as measured in the background of the images. For all parameters, we determined the relative changes between the standard- and small-voxel reconstructions.
Eq. 3In this equation, SDbg is the SD of the 18F-FDG concentration as measured in the background of the images. For all parameters, we determined the relative changes between the standard- and small-voxel reconstructions.
Patient Study
For quantitative analysis of the patient data, the same workstation was used as for the phantom study. Lesion volume was determined by taking the average lesion volume (based on isocontours at 50% of the maximum pixel value) derived from both reconstructed PET images. Only 18F-FDG PET–positive lesions in the chest and upper abdominal region with an average lesion volume of less than 3.0 mL (≈diameter, <18 mm) were included for analysis. Lesions were considered as 18F-FDG PET–positive when there was an increased uptake (visually assessed), compared with the mediastinal blood pool (21–23) on the standard-voxel reconstruction. A maximum of 3 lesions per patient was incorporated to avoid the impact of some patients with many small lesions. In these cases, the 3 smallest lesions were selected. In total, we included sixty-six 18F-FDG PET–positive lesions, located, respectively, in the mediastinum (n = 30), lung (n = 23), hilar region (n = 8), axilla (n = 3), and high abdominal region (n = 2). A subanalysis was performed for lesions less than 0.75 mL (≈diameter, <11 mm).
For each lesion, we measured the mean and maximum standardized uptake values (SUVmean and SUVmax, respectively), where the mean value was again based on the isocontour at 50% of the maximum pixel value. We performed background measurements in the mediastinal blood pool, by drawing an ROI of approximately 400 mm2. This way, the SUVmean (SUVbackground) and SD in this background were determined. Subsequently, SNRmean, SNRmax, and background noise were calculated in a similar way as for the phantom study. For all parameters, we determined the relative changes between the standard- and small-voxel reconstructions.
For a visual analysis, 3 experienced nuclear medicine physicians, who were unaware of the study purpose, performed a side-by-side analysis of the standard- and small-voxel reconstructed images. For the 66 selected lesions, the physicians had to rank their preference based on lesion sharpness, lesion contrast, and diagnostic confidence.
Statistical Analysis
We used the Wilcoxon signed-rank test to compare SUVmean, SUVmax, SNRmean, and SNRmax measurements between the standard- and small-voxel reconstructions. Furthermore, we performed linear regression analysis to determine correlations between lesion volume and relative changes in SUVmean, SUVmax, SNRmean, and SNRmax. In this perspective, we performed the F test and calculated Pearson correlation coefficients. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Phantom Study
Figure 1 shows illustrations of the NEMA phantom and microphantom that were reconstructed using standard and small voxels. When Figures 1A and 1B are compared, it is clearly visualized that the 2 smallest spheres of the NEMA phantom have an enhanced contrast on the small-voxel reconstruction (Fig. 1B). Furthermore, as can be seen in Figure 1D, the contrast of the small spheres in the microphantom is clearly increased on the small-voxel reconstruction, compared with the standard-voxel reconstruction (Fig. 1C). This particularly pertains for the smallest phantom sphere (4 mm) of the microphantom, which could not be distinguished from the background on the standard-voxel reconstruction, whereas it is clearly visualized on the small-voxel reconstruction. Background noise levels in the NEMA phantom were 5.1% and 7.5% for the standard- and small-voxel reconstruction, respectively. For the microphantom, noise levels for these reconstructions were 4.0% and 5.6%, respectively.
18F-FDG PET images of NEMA phantom (A and B) and microphantom (C and D) using standard-voxel reconstruction (A and C) and small-voxel reconstructions (B and D). Sphere sizes for NEMA phantom were 10, 13, 17, 22, 28, and 37 mm, inner diameter, and sphere sizes for microphantom were 4, 5, 6, and 8 mm, inner diameter. For all spheres with diameter of 13 mm or less, contrast is clearly increased using small-voxel reconstruction. Moreover, smallest microphantom sphere cannot be distinguished from background on standard-voxel reconstruction (C), yet it can be detected on small-voxel reconstruction (D).
The impact of the small-voxel reconstruction on CRCmean, CRCmax, SNRmean, and SNRmax is summarized in Table 1. As presented in this table, for all parameters we found increases for the small spheres (≤13 mm) using the small-voxel reconstruction. The relative increases were the highest for the smallest spheres (≤6 mm). In particular for the 5- and 6-mm spheres, we found relative increases of more than 100% for CRCmax and SNRmax. The 4-mm microphantom sphere could not be distinguished from the background on the standard-voxel reconstruction (listed as not applicable). For the larger spheres (≥17 mm), decreases in SNRmean and SNRmax were approximately 30%. These decreases were related to the increase of noise on the small-voxel reconstruction. However, the detection of these spheres on the small-voxel reconstruction was not compromised because the SNRs were still quite large (>60). Furthermore, the CRCs remained constant for the larger spheres as presented in Table 1.
CRCmean, CRCmax, SNRmean, and SNRmax for 10 Phantom Spheres for Both Voxel Reconstructions, Including Relative Changes (%)
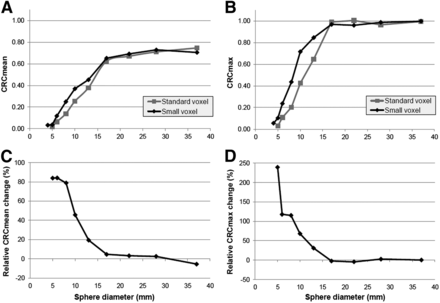
Figure 2 presents the CRCmean and CRCmax for the 10 spheres (the smallest 4 from the microphantom) for both reconstruction types. Furthermore, it includes the relative changes in CRC between the standard- and small-voxel reconstruction. As can be seen in this figure, for the spheres 13 mm or less, both the CRCmean and the CRCmax were higher on the small-voxel reconstruction, inducing relative increases up to 80% for the CRCmean and 200% for the CRCmax.
CRCmean (A) and CRCmax (B) for phantom spheres using standard- and small-voxel reconstructions, with relative changes (%) for both parameters presented in plot C and D. For small spheres (≤13 mm), we found increases for CRCmean and CRCmax using small-voxel reconstruction, with highest relative increases for 5- and 6-mm small spheres. As 4-mm small spheres could not be distinguished from background on standard-voxel reconstruction, no relative CRC changes were determined.
Patient Study
Regarding quantitative analysis, the average lesion volume was 1.18 mL (diameter, ∼18 mm), with 21 lesions smaller than 0.75 mL (diameter, ∼11 mm). The average background noise was 19% higher on the small-voxel images (17% vs. 20% for standard- and small-voxel images, respectively).
The average changes in SUVmean and SUVmax we found using the small-voxel images were 17% (P < 0.001) and 32% (P < 0.001), respectively. These values increased up to 21% and 44%, respectively, for lesions less than 0.75 mL. The increases in SNRmean and SNRmax were 13% (P = 0.015) and 27% (P < 0.001), respectively. For lesions less than 0.75 mL, these values improved toward 23% and 46%. We found moderate correlations between lesion volume and relative changes in SUVmean, SUVmax, SNRmean, and SNRmax with Pearson correlation coefficients of −0.34, −0.38, −0.23, and −0.27, respectively. Nevertheless, changes in SUVmean, SUVmax, and SNRmax correlated significantly with lesion volume (P < 0.05). The correlation between lesion volume and relative change in SNRmean was nonsignificant (P = 0.07). Relative changes in SUVmax and SNRmax for all lesions, using the small-voxel reconstruction instead of the standard-voxel reconstruction, are presented in Figure 3.
Relative changes in SUVmax (A) and SNRmax (B) for all 66 included lesions using small-voxel reconstruction instead of standard-voxel reconstruction. Average changes in SUVmax and SNRmax across all lesions were 32% and 27%, respectively. For lesions smaller than 0.75 mL, we found average SUVmax and SNRmax increases of 44% and 46%, respectively.
Regarding visual analysis, the 3 physicians preferred the small-voxel images in, respectively, 64%, 77%, and 88% of cases (average, 76%). Furthermore, in 88% of cases, at least 2 physicians preferred the small-voxel images.
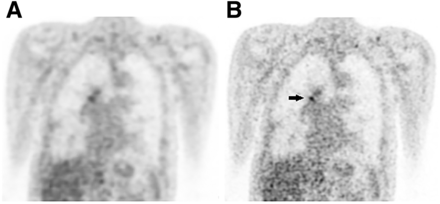
In Figures 4 and 5, 2 clinical examples are presented comparing the standard- and small-voxel reconstructed PET images. The SUVmax and SNRmax of the lung lesion, visualized in Figure 4, increased with 54% and 115%, respectively. For the lesion in the right hilar region, as can be seen in Figure 5, we found SUVmax and SNRmax increases of 46% and 77%, respectively.
Transverse 18F-FDG PET images using standard-voxel reconstruction (A) and small-voxel reconstruction (B). Lesion in left lung (volume, 0.68 mL) with SUVmax of 2.6 using standard-voxel reconstruction increased with 54% to 4.0 using small-voxel reconstruction. SNRmax increased with 115% (from 3.1 to 6.6). ROIs used for background measurements are illustrated by black circles.
Coronal 18F-FDG PET images with standard-voxel reconstruction (A) and small-voxel reconstruction (B). SUVmax of lesion in right hilar region (volume, 0.50 mL) with SUVmax of 3.0 using standard-voxel reconstruction increased with 46% to 4.4 on small-voxel reconstruction (black arrow). SNRmax increased with 77% (from 9.8 to 13.3).
DISCUSSION
In this investigation, we demonstrated that the detection of small lesions on a state-of-the-art TOF PET/CT device can be improved using reconstructions with smaller voxels. We found significant improvements in SUV and SNR, and moreover our physicians preferred the 18F-FDG PET images reconstructed with 2 × 2 × 2 mm voxels in most cases.
The CRC measurements in our phantom study for both standard- and small-voxel reconstructions are consistent with the results of Kolthammer et al. (16). Furthermore, the improvement of CRCs for small-voxel reconstructions implies an improved image resolution, which has a direct impact on the interpretation of clinical SUV measurements of small lesions.
The use of a small-voxel reconstruction also influences image quality in terms of image noise. With equal administered 18F-FDG dose and acquisition time, the small-voxel reconstruction implies less counts per voxel, and therefore the image noise intrinsically increases. Furthermore, image noise is influenced by the image reconstruction process and the use of different reconstruction settings such as relaxation and blob shape parameters (7,24,25). The increase in noise on the small-voxel reconstruction explains the 30% decrease in SNR we found for the large spheres (≥17 mm) in our phantom study. However, we showed that despite the increase in noise, the SNR of small spheres and lesions significantly improved using the small-voxel reconstruction on a state-of-the-art TOF PET/CT device.
For this study, we used default settings provided by the manufacturer, for both the standard- and the small-voxel reconstructions. Although it is expected that these settings are suitable for clinical PET/CT studies, these may not be the most optimal settings. Hence, optimization of the reconstruction settings such as the number of iterations, subsets, and blob and relaxation parameters could possibly further improve the detection of small lesions (26–28). Moreover, previous studies have shown the added value of modeling of the point-spread function for the detection of small lesions (13,14).
In addition, in this study we evaluated the small-voxel reconstructions on the phantoms with a sphere-to-background ratio of 10, according to EANM procedure guidelines (17). It would be valuable also to perform phantom studies with lower concentration ratios, for example, with a sphere-to-background ratio of 4, to study the impact of small-voxel reconstructions on the detectability of lesions with low contrast.
In this study, 3 nuclear medicine physicians were asked to compare the standard- and small-voxel reconstructed images and to give their preference. Before this study was executed, the physicians were not yet familiar with small-voxel reconstructed images. However, they all preferred the small-voxel reconstructed images in most of the cases, indicating that, in general, the higher image noise level on small-voxel images is accepted, with the advantage of improved small lesion detectability.
For the 3 physicians, the small-voxel image preference ranged between 64% and 88%. This disagreement can be explained by the subjective origin of the study, small differences in experience with small-voxel images, and the inclusion of some cases in which the difference in lesion detectability between standard- and small-voxel images was small, leading to some subjective random choices. However, the introduction of small-voxel images is subject to a learning effect and therefore we expect the preference to further increase after more experience with small-voxel reconstructions. An increase in physicians' experience will probably also reduce interobserver disagreement.
A limitation of this study is that we did not assess the clinical impact of the small-voxel reconstruction in terms of sensitivity and specificity, which was outside the scope of this study. However, we found improved CRCs and SNRs in small (phantom) spheres and higher SUVs and SNRs for small lesions. Therefore, we expect that cutoff values for small-lesion detection, for example, as published by Tournoy et al. (5) and Li et al. (29), will further increase for 18F-FDG PET studies acquired with state-of-the-art TOF PET/CT scanners, moreover for small-voxel reconstructions. Recently, this expectation was also discussed by Kolthammer et al. (16). Validation studies are necessary to determine sensitivity and specificity and define new cutoff values to distinguish benign from malignant small lesions using state-of-the-art TOF PET/CT.
CONCLUSION
On the basis of our study, it is expected that detection of small lesions using 18F-FDG PET improves using small-voxel reconstructions on a state-of-the-art TOF PET/CT system. Supported by a phantom study, there was a preference by the physicians toward 18F-FDG PET images reconstructed with 2 × 2 × 2 mm voxels and a profound increase in SUV and SNR for small lesions (<11 mm). Validation studies are necessary to determine the impact of small-voxel reconstructions in terms of sensitivity and specificity. Furthermore, new (probably higher) cutoffs and lesion-to-background values need to be defined, to distinguish benign from malignant small lesions using small-voxel reconstructions on a state-of-the-art TOF PET/CT device.
DISCLOSURE
This work was supported by a research agreement between the Department of Nuclear Medicine, Isala Hospital, and Philips Healthcare regarding new PET technologies. The content of the article was solely the responsibility of the authors. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jan. 22, 2014.
REFERENCES
- Received for publication October 16, 2014.
- Accepted for publication November 24, 2014.