Abstract
γ-cameras use flood-field corrections to ensure image uniformity during clinical imaging. A loss or corruption of the correction data of one head of a dual-head camera can result in an off-peak artifactual appearance. We present our experience with the occurrence of such an incident on a 67Ga scan. Methods: A patient was referred for a whole-body 67Ga scan to evaluate for causes of neutropenic fever. Whole-body planar and static images of the head, chest, abdomen, pelvis, and lower extremities in multiple projections were obtained. Results: Whole-body images showed decreased image quality on the anterior view obtained with detector 1 and an unremarkable posterior image obtained with detector 2. A problem with detector 2 was suspected, and additional static images were obtained after rotation of the detector heads. The posterior images taken with detector 1 showed photomultiplier tube outlines. The anterior images taken with detector 2 showed improved count and image quality. It was later found that the uniformity map for detector 2 had been lost and that this software malfunction led to the resulting imaging problem. Conclusion: When artifacts with an off-peak appearance are seen on scintigraphic images, evaluation of possible causes should include not only isotope window settings but also an incorrect or corrupted uniformity map.
Although current γ-camera systems use state-of-the-art strategies to ensure acquisition of optimal images, it is important that we, the professionals in the field, be vigilant in overseeing the status of our camera systems on a daily basis before any image acquisition is performed. In the case described here, a patient’s 67Ga scan was noted to have a classic off-peak appearance that was finally found to be due to a corrupted uniformity map.
CASE REPORT
This was a case of a 62-y-old man with a history of AIDS who presented at a community hospital for evaluation of neutropenic fever. The referring service requested a 67Ga scan. Whole-body images were obtained in anterior and posterior projections 48 h after the administration of 222 MBq (6 mCi) of 67Ga-citrate. Additional images of the head, chest, abdomen, pelvis, and lower limbs were obtained. Imaging was performed on a dual-head E.CAM γ-camera (Siemens). Simultaneous anterior and posterior projections were acquired. Whole-body images were acquired in a 256 × 1,024 matrix for a length of 200 cm at 8 cm/min. Limited views of the head, chest, abdomen, pelvis, and extremities were acquired in a 256 × 256 matrix for 5 min or 300,000 counts.
Anterior and posterior whole-body and static images demonstrated normally distributed radiotracer activity with no suggestion of infection. However, the patient’s images also revealed another interesting, unrelated finding. The whole-body study showed an appreciable difference in image quality between the anterior and posterior images. An apparent reduced number of counts and poor image quality could be seen in the anterior whole-body image (Fig. 1).
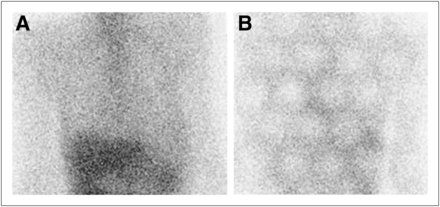
Anterior and posterior whole-body images obtained with detectors 1 and 2, respectively, showing marked decrease in counts and image quality on anterior projection. No distinct PMT outlines are detectable.
A problem with detector 1 was suspected. Additional static images were then obtained after rotation of the detector heads. The anterior static images showed improved image quality with use of detector 2, whereas the posterior images taken with detector 1 showed degraded image quality, with photopenic defects corresponding to the photomultiplier tube outlines (Fig. 2). No similarly distinct PMT outlines could be appreciated on the anterior whole-body image obtained with detector 1 (Fig. 1).
Static images after detector rotation. (A) Anterior view of the chest and upper abdomen showing good-quality image with use of optimally functioning detector 2. (B) Posterior view showing PMT outlines with use of problematic detector 1.
It was later discovered that the correction floods for detector 1 had been corrupted, causing the off-peak appearance even though the proper 67Ga window had been configured and the medium-energy collimator was in place. This problem was resolved the same day after on-site vendor service had been provided and appropriate corrections had been made. No faulty photomultiplier tubes were found at the time of servicing. It was also verified that the appropriate correction flood for 67Ga had been used before the study began.
The individual photomultiplier tubes were not seen on the whole-body images because of the moving detector heads; the outlines were blurred and the photomultiplier tube defects were filled in as the counts were being acquired, stored, and processed. Closer scrutiny revealed a subtle photomultiplier tube pattern over the patient’s head in the anterior view (Fig. 1) because detector 1 was stationary for several seconds at the commencement of image acquisition before moving continuously over the whole body.
For individual static planar imaging (Fig. 2), the detector was stationary over the field of view during the entire acquisition and hence clearly showed the photomultiplier tube outlines (1).
DISCUSSION
In nuclear medicine, optimum-quality images are of paramount importance in correct interpretation of any radionuclide study. Poor-quality images can result from various technical problems. Images should be reviewed by technologists and physicians (including planar statics, cine images, projections, and sinograms as needed) before the patient departs from the nuclear medicine suite, to ensure that the patient is promptly rescanned if necessary.
In this case, the classic off-peak appearance was caused by a corrupted uniformity map (also referred to as a correction flood). Without uniformity correction, photomultiplier tube–shaped defects result in degraded spatial resolution. These were apparent despite the fact that no faulty tubes were found at the technical service inspection. The remarkably low detected photon count was probably due to the uniformity correction strategy used by manufacturers. Correction circuit methodology varies widely among manufacturers. A flood source image often provides simple and effective evaluation of the correction circuits’ function (2). These flood field images are typically stored and used to adjust subsequent clinical images appropriately and accordingly with changes in energy and window settings. Most modern γ-cameras have intrinsic hardware designed to correct for field nonuniformity, but functioning software and available correction data are also essential for proper operation (1). Correction floods and uniformity maps are the same for current γ-camera systems and are usually acquired as intrinsic floods. Typically, no extra uniformity corrections are required for detectors with a collimator (extrinsic floods). In this case, the possibility that energy correction tables were corrupted or lost was excluded by the manufacturer’s electronic photomultiplier services. Energy correction tables are built into the electronic system in the manufacturer’s machines.
The other, more classic, scenario causing such an appearance (and one that should also be considered) is when the camera has been incorrectly peaked. A common example is when a medium- or high-energy tracer (such as 67Ga) is used but the camera is incorrectly peaked in a 99mTc window centered at 140 keV. This error is common simply because of the high proportion of 99mTc-based tracers relative to other isotopes in daily practice. Additionally, photomultiplier tubes can drift over time because of fluctuations in the line voltage and other conditions and need to be checked regularly. Other possible hardware-related causes are malfunctioning power boards (3) and photomultiplier tubes (4). Therefore, checking the energy window in spectral display mode before image acquisition is imperative in daily clinical routine. For cardiac SPECT studies, the sinograms should also be evaluated.
When the energy window is off-peak, photomultiplier tube–shaped defects may be apparent, depending on how significant the shift magnitude is. In addition, a portion of the Compton scattered photons is inappropriately detected instead of being rejected by the incorrectly set energy window. Both factors result in degraded spatial resolution. Remarkably low counts are another common phenomenon seen in static planar images with off-peak energy windows because most of the desired photons with the appropriate peak energy are rejected by the wrongly set energy window.
CONCLUSION
This patient’s examination was noted to have a classic off-peak appearance that was finally found to be due to a corrupted uniformity map. This appearance should alert technologists and physicians to check not only for incorrect isotope window settings but also for a possibly incorrect or corrupted correction flood or uniformity map. If any image artifacts are seen, immediate attention should be paid to the window settings to see if the camera was peaked properly, use of the correct isotope-specific uniformity flood should be verified, and the integrity of the correction floods should be evaluated. For cardiac SPECT studies, the sinograms should also be evaluated (3). Appropriate servicing should immediately be performed and patients rescanned as necessary.
Acknowledgments
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Sep. 27, 2012.
REFERENCES
- Received for publication September 19, 2011.
- Accepted for publication May 18, 2012.