Abstract
Our objective was to compare the stability of Kinevac when reconstituted with sodium chloride injection, USP, 0.9%, versus the manufacturer's recommended sterile water for injection, USP, and to determine the effects on stability of deviating from the manufacturer's recommended methods of product preparation. Methods: Kinevac was reconstituted with either sterile water or 0.9% sodium chloride. Triplicate high-performance liquid chromatography was performed on each vial of reconstituted sample at time zero and at time zero plus 8 h. The concentration of each sample, as measured by the peak area, was recorded at each time point. The process was repeated over 4 consecutive days. Results: Kinevac reconstituted with sterile water resulted in the recovery of 89.73% of the time zero concentration after 8 h. Kinevac reconstituted with 0.9% sodium chloride resulted in chemical stability of the injection, with 80.05% recovery of the time zero value after 8 h. Conclusion: Kinevac is more stable when reconstituted with sterile water than when reconstituted with 0.9% sodium chloride. Kinevac should be reconstituted with sterile water for injection as per the manufacturer's instructions.
Sincalide for injection (Kinevac; Bracco Diagnostics Inc.) is a cholecystopancreatic gastrointestinal hormone peptide for parenteral administration. The agent is a synthetically prepared C-terminal octapeptide of cholecystokinin. Sincalide is designated chemically as l-aspartyl-l-tyrosyl-l-methionylglycyl-l-tryptophyl-l-methionyl-l-aspartylphenyl-l-alaninamide hydrogen sulfate (ester) (1–3) (Fig. 1).
Sincalide peptide structure.
Kinevac is commonly used in nuclear medicine hepatobiliary studies to stimulate gallbladder contraction, thus emptying the organ and substantially reducing its size. The resulting evacuation of bile is similar to that occurring physiologically in response to endogenous cholecystokinin. This technique is useful for facilitating gallbladder uptake of radiotracer or for measuring gallbladder ejection fraction. In nuclear medicine, Kinevac is frequently prepared by nonpharmacy personnel who are unfamiliar with the potential significance of deviating from manufacturer instructions. Personal experience of witnessing Kinevac preparation using sodium chloride injection, USP, 0.9%, and the unexpected absence of research on this issue was the impetus for this investigation. Our objectives were to determine the stability of Kinevac when reconstituted with 0.9% sodium chloride versus the manufacturer's recommended sterile water for injection, USP, and to determine the effects on stability of deviating from the manufacturer's recommended methods of product preparation.
MATERIALS AND METHODS
Kinevac was reconstituted with either sterile water or 0.9% sodium chloride. Duplicate high-performance liquid chromatography (HPLC) was performed on each vial of reconstituted sample at time zero and at the end of 8 h (4,5). The sample concentration at each time point was recorded as measured by the peak area. The process was repeated over 4 consecutive days. The study materials consisted of Kinevac for injection, sterile water for injection, 0.9% sodium chloride for injection, sincalide (97% [HPLC] powder [active ingredient of Kinevac]), 150 mM phosphate buffer, HPLC-grade methanol, N-propanol, and water.
Preparation of Solution
For each assay, 2 vials of Kinevac were reconstituted. One vial was reconstituted with 5 mL of sterile water, and 1 with 5 mL of 0.9% sodium chloride. Each vial was mixed using a vortex machine set to low speed for 15 min to ensure complete reconstitution (4,5).
HPLC Analysis
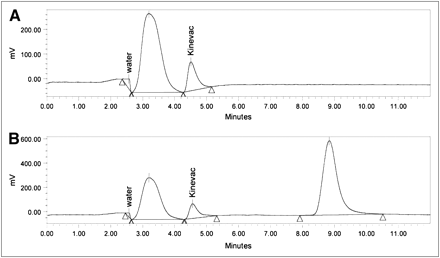
The stability of Kinevac was monitored by reverse-phase HPLC analysis; the results were calculated on the basis of peak areas (6). Samples were analyzed using an HPLC system (Waters) equipped with an electrochemical detector (Waters), an automatic sampler, and a gradient pump, with system operation and data analyses controlled by chromatography management software (Empower; Waters). A Nova-Pak C18 reverse-phase analytic column (4 μm, 3.9 × 150 mm; Waters) and a guard column were used for separation of the Kinevac active ingredient from the components added during the manufacturer's lyophilization process. The electrochemical detector was set at 900 mV for signal detection. The injected sample volume was 75 μL. A flow rate of 0.5 mL/min was used throughout the run. The total run time was 12 min per injection, including reequilibration of the column. The mobile phase consisted of 80% phosphate buffer and 20% N-propanol using an isocratic procedure. We calibrated the signal detection of the instrument using sincalide (Sigma), solubilized in water, as a reference standard to determine the retention time of the Kinevac active ingredient for identification purposes. This analysis method is based on a published method (7). Example chromatographs are provided in Figure 2.
Representative automatically scaled chromatographs with water and Kinevac peaks labeled. Triangles represent peak boundaries for area integration. (A) Kinevac reconstituted in sterile water and (B) Kinevac reconstituted in 0.9% sodium chloride. Third peak in B results from detection of sodium chloride in 0.9% sodium chloride (unlabeled).
Data Collection
Drug concentrations at time zero and at 8 h were recorded using the peak area measured by the electrochemical detector to monitor the active ingredient and determine drug stability.
RESULTS
Kinevac reconstituted with sterile water resulted in a measurement of 89.73% (±2.49) of the injected Kinevac time zero value (defined as 100%) after 8 h in solution. Kinevac reconstituted with 0.9% sodium chloride resulted in a measurement of 80.05% (±4.07) of the injected Kinevac time zero value after 8 h in solution. Results of the 4 tests are provided in Table 1. An unpaired t test results in a 2-tailed P value equal to 0.0126. The mean difference between sterile water– and 0.9% sodium chloride–solubilized Kinevac is 9.68%, with a 95% confidence interval of 2.94%−16.41%.
Stability 8 Hours After Reconstitution Measured as Percentage of Time Zero Kinevac Concentration Remaining
DISCUSSION
The stability of Kinevac prepared with both sterile water and 0.9% sodium chloride was calculated using HPLC analyses. A signal value that measured the amount of drug present was abstracted at time zero and at 8 h, with repeated experiments recorded over 4 consecutive days. The quantitative values for Kinevac reconstituted with sterile water and Kinevac reconstituted with 0.9% sodium chloride were compared to evaluate the stability of the drug after 8 h. On the basis of the null hypothesis that the reconstitution fluid does not affect chemical stability, the unpaired t test showed that the decreased stability of Kinevac was statistically significant.
CONCLUSION
Kinevac is more stable when reconstituted in sterile water than when reconstituted in 0.9% sodium chloride. Kinevac should be reconstituted in sterile water as per the manufacturer's instructions.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication January 15, 2008.
- Accepted for publication January 6, 2009.