Abstract
A cardiac emergency in SPECT/CT and PET/CT occurs infrequently but necessitates prompt recognition and an appropriate response. The emergence of 18F-based myocardial perfusion radiopharmaceuticals is anticipated to increase the use of cardiac stress testing; therefore, it is crucial for personnel, including nuclear medicine technologists in PET departments, to be equipped with proper training and competency to identify and manage deteriorating cardiac patients or emergency cardiac events. This article provides insight into the foundation principles of both cardiac stress testing and the use of adjunctive medications to manage patients after stress. The acute deteriorating nuclear cardiology patient is outlined, including recognizing crucial changes in vital signs and basic electrocardiogram interpretation. Key medications associated with an emergency response are detailed. Armed with these tools, nuclear medicine technologists can more confidently care for high-risk nuclear cardiology patients.
SPECT has been the primary method of evaluation of myocardial perfusion, despite its well-recognized shortcomings (1). In part because of relatively high cost and short half-lives, 13N-ammonia and 82Rb-chloride PET myocardial perfusion have had limited infiltration and assimilation in clinical practice. The emergence of 18F-based myocardial perfusion radiopharmaceuticals has renewed interest in myocardial perfusion stress testing in PET (1).
A recently published phase III study showed 18F-flurpiridaz PET myocardial perfusion imaging as a promising novel tracer for coronary artery disease detection, offering superior sensitivity, image quality, diagnostic certainty, and reduced radiation exposure compared with 99mTc-labeled SPECT myocardial perfusion imaging (2). 18F-flurpiridaz is well suited to exercise myocardial perfusion studies because of its favorable half-life and high myocardial extraction efficiency. Unlike 82Rb-chloride, which has a very short half-life (75 s), or 13N-ammonia (10-min half-life), which is limited to facilities with on-site cyclotrons, 18F-flurpiridaz has a half-life of 109 min. This extended half-life affords sufficient time to conduct treadmill or other forms of exercise stress testing (vs. pharmacologic stress testing), where the radiotracer can be injected at peak exercise and imaging can occur without the logistic constraints posed by shorter-lived tracers. Additionally, 18F-flurpiridaz demonstrates a high first-pass extraction fraction of 94%, enabling accurate myocardial blood flow quantification even at high coronary flow rates during exercise, offering superior image quality, diagnostic accuracy, and flexibility in clinical workflows compared with 82Rb-chloride or 13N-ammonia (1–3).
Indeed, the growing number of cardiac patients undergoing PET highlights the need for formal strategies to equip staff with the skills to identify and manage acute cardiac events. These events may arise de novo or during stress testing conducted as part of PET procedures. Given the vulnerability of this patient population, it is essential that all personnel, technologists, nurses, and support staff receive appropriate training in recognizing early signs of cardiac distress, performing basic life support, and activating emergency protocols. This training should include a strong understanding of cardiovascular physiology, common cardiac conditions, and potential complications linked to stress testing. Standardized education programs and regular emergency simulations can significantly enhance the department’s readiness to manage acute cardiac events, ultimately improving patient safety and outcomes in this high-risk environment.
With the introduction of 18F-flurpiridaz PET myocardial perfusion imaging into mainstream clinical practice, it is expected there will be an increase in stress testing being done in the PET departments. Exercise and pharmacologic stress testing inherently involve at-risk cardiac patients who may experience acute complications such as arrhythmias, ischemia, or hemodynamic instability during or after physical exertion. The incorporation of exercise stress protocols in 18F-flurpiridaz PET necessitates the development of robust strategies within PET departments to manage potential adverse cardiac events.
THE DETERIORATING PATIENT
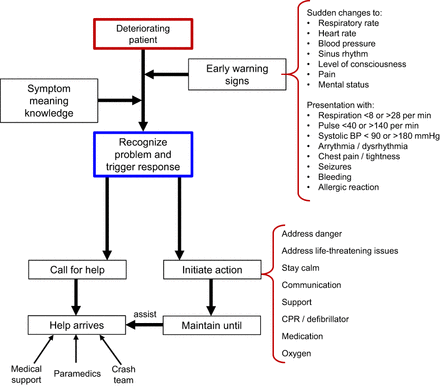
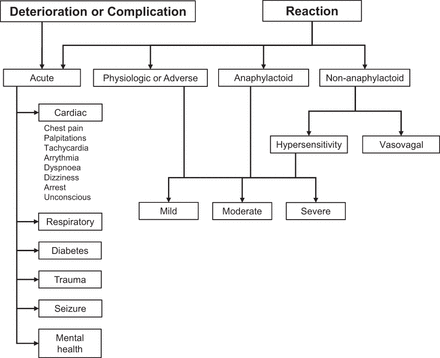
Stress testing is generally considered safe; however, it is not entirely without risk. Exercise stress has the lowest (0.015%–0.04%) rate of major stress-related adverse events when compared with pharmacologic interventions such as dobutamine (0.18%–0.2%) or vasodilation with adenosine or dipyridamole (0.07%–0.08%) (4). Recognizing the deteriorating patient relies on a degree of intuition because patient interactions can be fleeting at times, which skews any reference or baseline characteristics. Furthermore, patient interactions can be shaped by social, emotional, and cultural factors that demand a degree of acuity or competence (5,6). For cardiac patients, during stress testing and imaging, an electrocardiogram (ECG) monitor might provide objective evidence of a patient’s changing condition. There are also several features or symptoms that provide clues to changes in patient condition (Fig. 1). Most notably, a significant change from baseline in respiratory rate, heart rate, or blood pressure; new or worsening symptoms of discomfort such as in the chest, neck, or shoulders; or a change in level of consciousness requires immediate attention (7). More specifically, a respiratory rate below 8 or above 28 per minute, a heart rate below 40 or above 140 beats/min, a systolic blood pressure below 90 or above 180 mm Hg, and arrythmia warrant attention (7). Medical emergencies are most likely associated with one of the following scenarios in nuclear cardiology (Fig. 2): common symptoms in response to pharmacologic stress (e.g., breathlessness, chest pain, palpitation, flushing, and vasodilator stress, also often causing symptoms of nausea and headache); complications associated with cardiac stress testing (exercise or pharmacologic) (e.g., angina, pulmonary edema, arrhythmia, acute coronary syndrome or myocardial infarction, and cardiac arrest); patient deterioration associated with underlying cardiac pathology (e.g., acute hypertensive or hypotensive state); or adverse drug reactions or drug–drug interactions associated with pharmacologic stress (e.g., hypersensitivity).
A flowchart of basic principles of recognizing and responding to deteriorating cardiac patient. BP = blood pressure; CPR = cardiopulmonary resuscitation. (Adapted with permission of (8).)
Summary flowchart of most likely urgent or emergency patient scenarios encountered among cardiac stress patients in PET. (Adapted with permission of (7).)
Nuclear medicine technologists should undergo regular recertification in first aid with advanced life support (cardiopulmonary resuscitation). This includes the capability for manually evaluating the patient’s vital signs.
STRESS TESTING
Interventional medications are used to evoke a specific physiologic response and include myocardial perfusion SPECT or PET, stress echocardiography, cardiac CT, and cardiac MRI. Adjunctive medications are also used in nuclear cardiology to respond to a patient’s condition during a procedure (e.g., nitroglycerin, salbutamol, and aminophylline). The nature of cardiac patients and the use of pharmacologic or exercise stress means that it is not uncommon for adjunctive medications to be required (8–10). Although exercise is generally considered the preferred cardiac stress testing method, exercise is not always ideal or possible, in which case pharmacologic stress testing can be used. Broadly, there are 2 approaches to pharmacologic stress testing: vasodilators and dobutamine as a positive chronotropic and inotropic agent, with or without atropine augmentation. Stress testing protocols combining pharmacologic agents and exercise are less commonly performed and have sparse validations on diagnostic efficacy and safety.
Adenosine, regadenoson, and dipyridamole have a direct and potent vasodilatory effect on coronary blood flow, which exaggerates the regional myocardial blood flow differences supplied by normal and diseased vessels (8–10). Dobutamine is a positive chronotropic and inotropic agent that increases heart rate and cardiac workload, increasing myocardial oxygen demand so it is possible to induce myocardial ischemia (8–10).
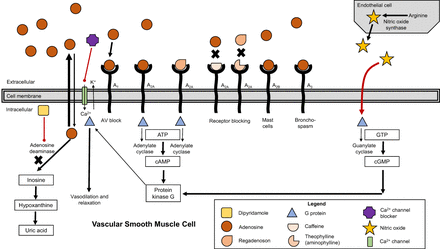
It is important to understand the basic pharmacology of these medications so that response in an emergency is intuitive. For vasodilators acting on vascular smooth muscle cells (Fig. 3), vasodilation is caused by agonism of the A2A adenosine receptors. Importantly, agonism of A1 and A3 can cause bronchoconstriction, and the adverse effects of nonselective adenosine and dipyridamole are largely overcome by the A2A-selective regadenoson (8–10). Exogenous adenosine acts directly on the A2A receptor via an adenylate cyclase–stimulating G protein to produce coronary and peripheral vasodilation. Dipyridamole increases the bioavailability of endogenous adenosine by antagonizing adenosine metabolism, which increases the availability of adenosine. The short half-life of adenosine (on the order of 10 s) means adverse effects can be managed with cessation of the infusion. Dipyridamole reversal with aminophylline is a misnomer because aminophylline does not change the action of dipyridamole. Instead, it blocks the action of adenosine at the receptor sites. The active drug (aminophylline is a prodrug) is theophylline, which is similarly structured to caffeine. Caffeine and theophylline have greater selectivity for antagonizing receptors A1 and A2A, but theophylline has 3–5 times higher potency than caffeine (8–10). Regadenoson selectively agonizes receptor A2A and thus avoids, in theory, the adverse effects associated with agonism of the other receptor subtypes. Because selectivity generally means lower affinity or potency for other receptors rather than no activity, regadenoson can produce adverse effects similar to those of adenosine, including atrioventricular (AV) block and bronchoconstriction.
Schematic representation of adenosine receptor action to cause vasodilation of vascular smooth muscle. ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate; cGMP = cyclic guanosine monophosphate; GTP = guanosine triphosphate. (Adapted with permission of (8).)
Clearly, adenosine can cause AV block via receptor A1, but because of its very short half-life, this is almost never of clinical significance. Contraindications for adenosine and dipyridamole include patients with symptomatic sick sinus syndrome, advanced heart blocks, Mobitz type I second- or third-degree AV block, and asthma or a history of bronchospasm (8,10).
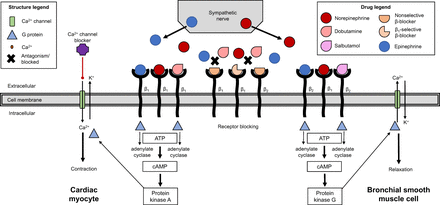
For dobutamine, the inotropic and chronotropic action in the cardiac myocyte is agonized in a similar fashion to endogenous norepinephrine released from the sympathetic nerve (Fig. 4). The endogenous norepinephrine and exogenous dobutamine can couple with β1 receptors, which, via adenylate cyclase–stimulating G protein, drive increased intracellular calcium that produces both an increased force and an increased rate of contraction (8–10). The selectivity for β1 receptors limits any bronchoconstriction that might occur with β2 receptor antagonism. This response from both exercise and dobutamine can be antagonized by a β-blocker. Adverse reactions to dobutamine can be managed with a fast-acting β-blocker. Calcium channel blockers can also antagonize the inotropic and chronotropic contraction response. In contrast to the action of dobutamine on β1 receptors, β2 receptor agonism by salbutamol in bronchial smooth muscle decreases intracellular calcium to cause bronchodilation and bronchial relaxation (Fig. 4). Salbutamol mimics endogenous norepinephrine by coupling with β2 receptors via a adenylate cyclase–stimulating G protein (8).
Schematic representation of adenosine receptor action to cause vasodilation of vascular smooth muscle. ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate; cGMP = cyclic guanosine monophosphate; GTP = guanosine triphosphate. (Adapted with permission of (8).)
ECG INTERPRETATION
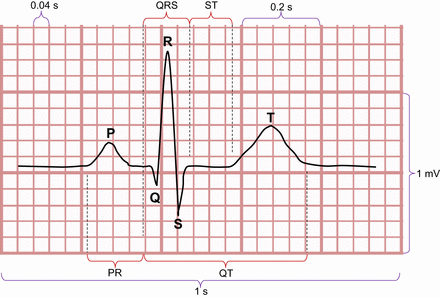
Most nuclear medicine technologists will be familiar with a single-lead ECG trace typically used for gating during imaging. Although these traces might also allow monitoring of patients for changes in heart rate or arrhythmia—for example, ectopic beats or clinically more sinister arrhythmia such as atrial fibrillation (AF), ventricular tachycardia (VT), and ventricular fibrillation (VF)—they do not provide the detail typical of a 12-lead ECG. The principles of the ECG have been previously described (11), and revision of those principles will enhance the usefulness of the following discussion (Fig. 5). The standard 12-lead ECG uses 10 electrodes producing 3 limb leads (I, II, and III), 3 augmented limb leads (aVR, aVL, and aVF otherwise known as augmented vector right, augmented vector left, and augmented vector foot, respectively), and 6 precordial or chest leads (V1–V6). The impulse that travels toward an electrode is recorded as an upright signal in that lead, whereas the impulse traveling away from an electrode is recorded as an inverted signal. The standard paper speed is 25 mm/s; therefore, a single small square equals 40 ms and a single large square (5 small squares) equals 200 ms. The PR interval represents the onset of atrial depolarization until the onset of ventricular depolarization, the ST segment is the period when the ventricles are depolarized, the T wave is the ventricular repolarization, and the QRS complex corresponds to ventricular depolarization.
Schematic representation of anatomy of ECG.
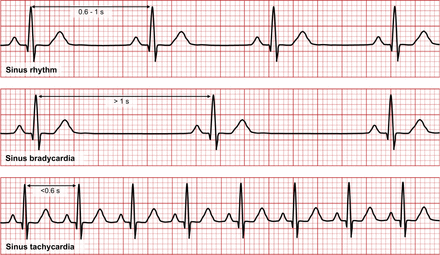
A normal sinus rhythm in healthy adults has a heart rate between 60–100 beats/min with sinoatrial (SA) node pacing (Fig. 6). Sinus bradycardia is when the SA node pacing produces slow heart rates (<60 beats/min) and sinus tachycardia when SA pacing produces a faster heart rate (>100 beats/min). Individuals with high levels of cardiopulmonary fitness, such as athletes, often exhibit a physiologically normal sinus bradycardia at rest. During exercise stress testing, however, their heart rate increases appropriately in proportion to the exercise workload, reflecting a normal physiologic response. There are a wide variety of pathologies that alter ECG rhythm, and an examination of all of these is beyond the scope of this article. So that nuclear medicine technologists can identify and respond to ECG changes among nuclear cardiology patients, the more common rhythm changes will be briefly outlined. These rhythms may come to technologists’ attention because they interfere with quality gated imaging.
Representation of ECG appearances of normal sinus rhythm (top), sinus bradycardia (middle), and sinus tachycardia (bottom).
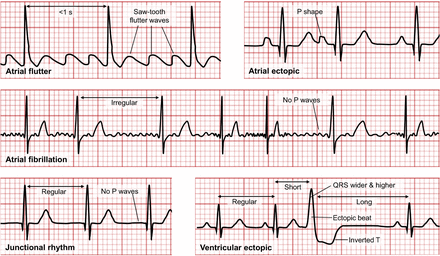
Atrial flutter occurs when the atria beat faster and out of synchrony with the ventricles (Fig. 7). AF occurs when the atria do not contract properly and results in irregular ventricular contractions and an absent P wave. An absent P wave is also characteristic of junctional rhythms in which AV pacing is slower than the SA node but faster than ventricular pacing. Premature atrial contraction occurs with atrial ectopics that have altered the shape of the P wave. Premature ventricular contractions create ectopic beats or a QRS complex that occurs earlier than expected and is typically wider and of higher voltage (amplitude) with an obscured P wave and inverted T wave.
Representation of ECG appearances of atrial flutter, atrial ectopic, AF, junctional rhythm, and ventricular ectopic.
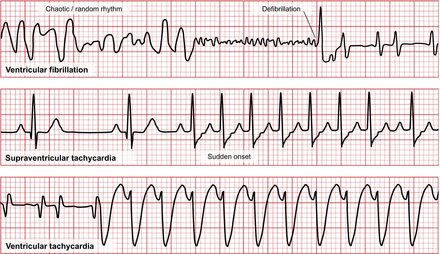
VF results from uncoordinated ventricular contractions and is a serious condition resulting in unconsciousness and needing immediate cardiopulmonary resuscitation or defibrillation (Fig. 8). Paroxysmal tachycardias literally mean sudden onset of a burst of tachycardia typically lasting seconds to minutes. They are classified by excitation origin as either ventricular tachycardia or supraventricular tachycardia (SVT).
Representation of ECG appearances of VF (top), SVT (middle), and VT (bottom).
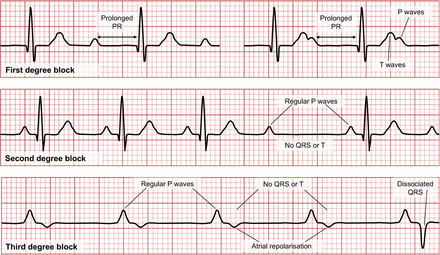
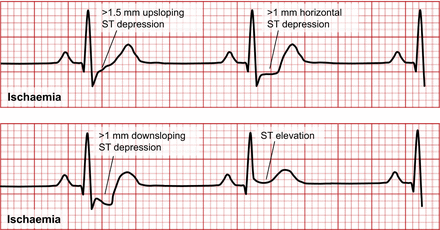
Sick sinus syndrome results when an SA node dysfunction slows impulse generation. AV block results from dysfunction of conduction between atria and ventricles and can be induced by adenosine (Fig. 9). First-degree block is characterized by a prolonged PR interval (>0.2 s). Second-degree block is generally associated with a normal P wave and PR interval but dropped ventricular contraction (absent QRS). Third-degree block or complete heart block has a dissociation between the P wave and QRS. Left bundle branch block (LBBB) produces a deep and broad S wave or QS complex on leads V1 and V2 and a broad positive R wave on leads V5 and V6. Both appearances can also result in ST or T changes—a possibility that is important to consider in stress test–induced ischemia (Fig. 10).
Representation of ECG appearances of first-degree block (top), second-degree block (middle), and third-degree block (bottom).
Representation of ST segment changes on ECG in ischemia with increasing severity from top left (upsloping ST depression) to bottom right (ST elevation).
CRASH CART
In nuclear cardiology, there are a few types of likely emergencies to generally consider. Cardiovascular emergencies, such as cardiac arrest, angina, arrhythmias, and hypertension or hypotension, can occur in any patient (individuals with coronary artery disease or structural heart disease are particularly at risk). Respiratory emergencies may arise in nuclear cardiology because of stress testing and preexisting conditions (e.g., asthma and chronic obstructive airways disease) and adverse reactions to interventional medications (e.g., dipyridamole). Endocrine emergencies are most likely associated with hypoglycemia in insulin-dependent diabetic patients who have fasted as part of test protocols.
Muscarinic antagonists such as atropine produce increased heart rate, vasoconstriction, hypertension, smooth muscle relaxation, and bronchodilation but can also result in decreased exocrine secretion, pupil dilation, increased intraocular pressure, cycloplegia, and increased body temperature (7,8). Atropine is used intravenously to treat sinus bradycardia and asystole. Atropine is also used quite routinely during dobutamine stress to assist in cardiac chronotropy (increasing heart rate) when the stress heart rate fails to reach 85% of the maximum predicted for age ([220 – age] × 85) at the peak dobutamine infusion rate or if peak dobutamine cannot be offered because of side effects.
Although insulin decreases blood glucose, glucagon increases it and can be administered (exogenous) as a nasal spray, intramuscularly, or intravenously to manage the unconscious hypoglycemic patient (8). Hypoglycemia is generally managed with glucose drinks or jelly beans.
Agonism of β1 receptors by either norepinephrine or epinephrine increases the heart rate, cardiac contraction force, cardiac output, and myocardial oxygen use but can cause arrhythmia (Fig. 4). Epinephrine is a nonselective β receptor agonist. Epinephrine can be used in any pulseless arrhythmia, in anaphylaxis, and to relax bronchial smooth muscle (7,8). Dobutamine is a selective β1 receptor agonist that is used to treat hypotension. Norepinephrine and dopamine can also be used for managing hypotension by causing vasoconstriction at higher doses (vasodilation and low doses).
Agonism of β2 receptors relaxes smooth muscle (bronchodilation, vasodilation), increases glycolysis in liver and muscle, and decreases histamine release. Salbutamol and salmeterol are selective β2 receptor agonists used as bronchodilators to treat bronchospasm (Fig. 4). Antagonists of the β-adrenergic receptors or β-blockers can be selective for β1 like atenolol and metoprolol to decrease heart rate, decrease contraction force, decrease oxygen demand, cause peripheral vasoconstriction, and produce bronchospasm (7,8). These β-blockers can be used to treat hypertension, arrhythmia, and angina.
Glyceryl trinitrate (GTN), commonly known as nitroglycerin, relieves angina through its ability to induce venodilation, reducing venous return (preload) and myocardial wall tension, as well as arterial dilation, lowering systemic vascular resistance (afterload). Additionally, GTN dilates coronary arteries, improving blood flow to ischemic myocardial regions. These effects restore the balance between myocardial oxygen supply and demand, alleviating angina symptoms within minutes (7,8). It is important to note that phosphodiesterase-5 inhibitors used for erectile dysfunction, such as sildenafil (Viagra; Pfizer), function by potentiating the effects of nitric oxide in the corpora cavernosa and so are contraindicated.
Furosemide is a loop diuretic that inhibits reabsorption of sodium, potassium, and chloride to increase urine volume. It has a potent and rapid onset of action that, combined with a short duration of effect, makes it ideal for managing acute hypertensive episodes (7,8). Because furosemide acts inside the lumen, it requires adequate glomerulus filtration for efficacy. Vasopressin is an antidiuretic hormone medication that increases water absorption and can be used to treat hypotension (8).
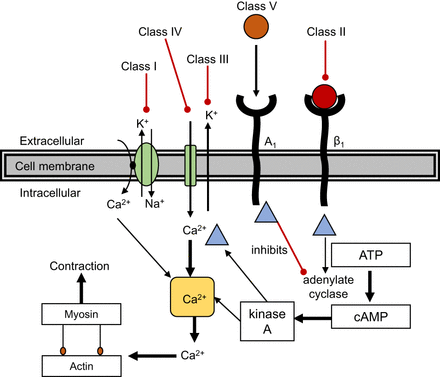
Antiarrhythmic medications are classified into 5 groups (Fig. 11). Class I antiarrhythmics are sodium channel blockers, which interfere with sodium influx to decrease AV conduction contractility (e.g., class Ia procainamide, used for VT, and class Ib lignocaine, used for VF, pulseless VT, and SVT) (7,8). Class II antiarrhythmics are β-blockers, which decrease AV conduction and contractility (e.g., atenolol and metoprolol, used for SVT). Class III antiarrhythmics are potassium channel blockers, which increase the action potential duration and decrease AV conduction (e.g., amiodarone, used for VF, VT, and SVT). Class IV antiarrhythmics are calcium channel blockers, which decrease the action potential duration and decrease contractility (e.g., verapamil, used for SVT) (7,8). Class V antiarrhythmics are purines, which block AV node conduction via A1 receptor action (e.g., adenosine, used for SVT). In an emergency situation, antiarrhythmic medications are designed to prevent life-threatening arrhythmia, prevent sudden cardiac death, and control the ventricular contraction rate, but paradoxically, all antiarrhythmic medications can worsen arrhythmia (7,8).
Schematic representation of 5 groups of antiarrhythmic medications (sodium channel, β receptor, potassium channel, calcium channel, and A1 agonism). ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate. (Adapted with permission of (8).)
CONCLUSION
Nuclear cardiology plays a critical role in managing patients with heart disease. Myocardial perfusion SPECT combined with increasing use of 18F-based perfusion PET increases the risk of experiencing an acutely deteriorating patient. Armed with the tools for detection and appropriate response, including pharmacology and ECG interpretation, nuclear medicine technologists can ensure patient safety throughout patient procedures.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive credit—number of credits awarded will be determined by the length of the article. Participants can access this activity through the SNMMI website (http://www.snmmilearningcenter.org) through XXX 2028. Additional details such as the number of credits issued per article, expiration dates, financial disclosure information, VOICE transcripts, and the process to earn CE credit can also be found in the SNMMI Learning Center.
Published online Mar. 4, 2025.
REFERENCES
- Received for publication January 1, 2025.
- Accepted for publication January 21, 2025.