Visual Abstract
Abstract
In a prospective clinical trial, [18F]fluoro-5α-dihydrotestosterone ([18F]FDHT), the radiolabeled analog of the androgen dihydrotestosterone, was used as a PET/CT imaging agent for in vivo assessment of metastatic androgen receptor–positive breast cancer in postmenopausal women. To our knowledge, this article presents the first report of PET/CT image–based radiation dosimetry of [18F]FDHT in women. Methods: [18F]FDHT PET/CT imaging was performed on a cohort of 11 women at baseline before the start of therapy and at 2 additional time points during selective androgen receptor modulator (SARM) therapy for androgen receptor–positive breast cancer. Volumes of interest (VOIs) were placed over the whole body and within source organs seen on the PET/CT images, and the time-integrated activity coefficients of [18F]FDHT were derived. The time-integrated activity coefficients for the urinary bladder were calculated using the dynamic urinary bladder model in OLINDA/EXM software, with biologic half-life for urinary excretion derived from VOI measurements of the whole body in postvoid PET/CT images. The time-integrated activity coefficients for all other organs were calculated from VOI measurements in the organs and the physical half-life of 18F. Organ dose and effective dose calculations were then performed using MIRDcalc, version 1.1. Results: At baseline before SARM therapy, the effective dose for [18F]FDHT in women was calculated as 0.020 ± 0.0005 mSv/MBq, and the urinary bladder was the organ at risk, with an average absorbed dose of 0.074 ± 0.011 mGy/MBq. Statistically significant decreases in liver SUV or uptake of [18F]FDHT were found at the 2 additional time points on SARM therapy (linear mixed model, P < 0.05). Likewise, absorbed dose to the liver also decreased by a small but statistically significant amount at the 2 additional time points (linear mixed model, P < 0.05). Neighboring abdominal organs of the gallbladder wall, stomach, pancreas, and adrenals also showed statistically significant decreases in absorbed dose (linear mixed model, P < 0.05). The urinary bladder wall remained the organ at risk at all time points. Absorbed dose to the urinary bladder wall did not show statistically significant changes from baseline at any of the time points (linear mixed model, P ≥ 0.05). Effective dose also did not show statistically significant changes from baseline (linear mixed model, P ≥ 0.05). Conclusion: Effective dose for [18F]FDHT in women before SARM therapy was calculated as 0.020 ± 0.0005 mSv/MBq. The urinary bladder wall was the organ at risk, with an absorbed dose of 0.074 ± 0.011 mGy/MBq.
The radiolabeled analog of the androgen dihydrotestosterone, [18F]fluoro-5α-dihydrotestosterone ([18F]FDHT), has been used as an imaging agent for in vivo assessment of androgen receptor expression (1,2). [18F]FDHT has been studied in prospective clinical trials as an imaging agent for castration-resistant prostate cancer in men since the early 2000 s (3–5). In contrast, prospective clinical trials have recently begun for [18F]FDHT imaging of metastatic androgen receptor–positive breast cancer in women (6,7).
The androgen receptor is expressed in most estrogen receptor–positive (ER+) breast cancers, and a prospective phase 2 therapeutic clinical trial investigating a novel selective androgen receptor modulator (SARM) in postmenopausal women with ER+ metastatic breast cancer was conducted at the Dana-Farber Cancer Institute (8,9). As part of that trial, we performed a prospective imaging substudy exploring the feasibility of [18F]FDHT PET/CT as an imaging biomarker for androgen receptor expression and evaluating response to SARM therapy (7–9). This article presents the [18F]FDHT image–based radiation dosimetry for the cohort of 11 women who participated in the [18F]FDHT PET/CT imaging substudy.
Zanzonico et al. published the human dosimetry of [18F]FDHT for 7 men with prostate cancer (10), and Beattie et al. studied [18F]FDHT pharmacokinetics (11). However, to our knowledge, there have been no published studies on the biodistribution and radiation dosimetry of [18F]FDHT in women.
MATERIALS AND METHODS
Patients and Study Design
As part of a prospective phase 2 therapeutic clinical trial investigating a novel SARM, enobosarm (G200802 [GTx, Inc.], NCT02463032), in postmenopausal women with ER+ metastatic breast cancer, 11 women participated in a single-site imaging substudy at Dana-Farber Cancer Institute from March 2017 through February 2018. The trial was conducted in full concordance with the principles of the Declaration of Helsinki and good clinical practice and was approved by the Dana-Farber Cancer Institute review board. All participants gave written informed consent.
Results from the clinical aspects of the imaging substudy (7) and the parent therapeutic clinical trial (8,9) have been reported. The major eligibility criteria for the phase 2 therapeutic clinical trial were postmenopausal women diagnosed with ER+/HER2-negative metastatic or locally recurrent advanced breast cancer with radiologic or clinical evidence of disease recurrence or progression within 30 d of randomization onto the therapeutic trial, at least 1 prior hormonal treatment but no more than 1 course of chemotherapy in the metastatic setting, tumor tissue from a biopsy or archival tissue available, bone-only nonmeasurable disease or measurable disease by RECIST 1.1, adequate organ function as shown by biomarkers in blood samples, Eastern Cooperative Oncology Group performance status 0 or 1, and no concurrent hormone replacement therapy. Participants were randomized 1:1 to receive 9 or 18 mg of enobosarm orally per day for up to 24 mo. Enobosarm specifically binds androgen receptors to promote agonist activity in ER+ breast cancer. Participants did not receive any hormonal therapy for the treatment of breast cancer other than the study drug on trial.
[18F]FDHT PET/CT Imaging
[18F]FDHT PET/CT scans were obtained before the start of SARM therapy (study time-point 0 [S0]) and at 42 ± 7 d (study time-point 1 [S1]) and 84 ± 7 d (study time-point 2 [S2]) after starting SARM therapy.
Whole-body PET/CT images were acquired from the mid-thigh through the vertex of the skull starting at 52 ± 6 min after intravenous administration of 310 ± 29 MBq (8.4 ± 0.8 mCi) of [18F]FDHT. [18F]FDHT was manufactured at the Brigham and Women’s Nuclear Medicine/Biomedical Imaging Research Core under investigational-new-drug application 122,852 using methods previously described (12,13). No patient preparation was required before the administration of [18F]FDHT. Participants were instructed to void before the start of PET/CT imaging.
All PET/CT images were acquired on GE Healthcare Discovery ST and Discovery MI scanners. PET emission data were acquired for 4 min per bed position in 3-dimensional acquisition mode with 23% overlap between bed positions. The PET images were reconstructed using ordered-subsets expectation maximization (OSEM) iterative reconstruction algorithms with corrections for attenuation, scatter, detector normalization, dead time, and the radioactive decay between the start and end of the PET imaging. PET reconstruction parameters were harmonized between the PET/CT scanners by imaging a phantom containing cylinders 12, 16, and 25 mm in diameter and an activity concentration ratio of 2.5 relative to the phantom background. The reconstruction parameters for each scanner were selected so that the mean pixel value in the background was with ±5% of the activity concentration in the phantom background and the interscanner difference in the mean pixel values in the cylinders was within ±1 SD times the standard deviation measured in the background.
The reconstructed PET image voxel values were converted to SUV, calculated as… Eq. 1
Eq. 1
where A0 was the administered activity, [A] was activity concentration per gram measured in the image voxels and corrected for the radioactive decay that occurred since the time of administration, body weightsubject was the weight of the participant in kilograms, and 1,000 was the constant to convert weight in kilograms to grams.
Low-dose CT images were acquired immediately before the PET acquisition on the PET/CT scanner and used for attenuation and scatter corrections and for anatomic localization of the organs in the PET/CT images during image analysis.
Image Analysis
Organ dose and effective dose calculations were performed using MIRDcalc software, version 1.1 (14), with time-integrated activity coefficients derived from [18F]FDHT PET/CT images. Time-integrated activity coefficients were calculated using an exponential decay model and the [18F]FDHT activity measured in source organs and regions in the PET/CT images (10,15). The source organs for [18F]FDHT dosimetry were the cerebellum, kidneys, liver, lungs, and spleen, and source regions were the remaining whole body, left ventricle blood pool, and red bone marrow.
The fraction of administered activity retained within organs at the time of imaging was… Eq. 2
Eq. 2
where [A]organ was mean activity concentration measured in a volume of interest (VOI) within the organ corrected for radioactive decay that occurred since the time of administration, and massorgan was the reference organ mass in grams for the reference adult female. The body weight for the reference adult female phantom was 58 kg (16). Direct image-based measurements of organ masses were not feasible because of the low contrast resolution of attenuation correction CT. Therefore, masses were estimated by scaling the reference mass of the organ by the ratio of the mass of the participant and the mass of the reference adult female.
The relationship between SUV and activity concentration was shown in Equation 1, and by substituting Equation 1 into Equation 2, the fraction of the administered activity in the organ was calculated as… Eq. 3
Eq. 3
where SUVorgan was the SUVmean measured in the VOIs within the organ.
The SUVmean was measured for VOIs within the whole body, cerebellum, kidneys, liver, lungs, and spleen on the [18F]FDHT PET/CT images. The SUVmean of heart chamber contents was also measured for a VOI within the left ventricle. The fraction of activity retained in these organs at the time of imaging was then calculated using Equation 3. The diameters of the VOI spheres were 1 cm in the cerebellum, 1.5 cm in the kidneys, 2 cm in the spleen and left ventricle blood pool, and 3 cm in the liver and lungs.
For 10 participants without bone disease, the fraction of activity in the red bone marrow was estimated using quantitative imaging as recommended by the 2010 European Association of Nuclear Medicine Dosimetry Committee (17). The fraction of activity in the red marrow was calculated as… Eq. 4
Eq. 4
where SUVmarrow was SUVmean in a spheric VOI 2 cm in diameter within a lumbar vertebral body and massmarrow was reference mass in grams for red marrow in the reference adult female.
For the participant with bone disease, the fraction of activity in the red bone marrow was estimated using the blood activity method (17), assuming no uptake in blood cells and assuming equilibrium between blood plasma and red marrow extracellular space. Therefore, the fraction of activity in the red marrow was calculated as… Eq. 5
Eq. 5
where SUVblood was SUVmean in a cylindric VOI of 1-cm diameter and 5-slice or about 2 cm height within the descending aorta in the thorax. RMECFF was the red marrow extracellular fluid fraction, HCT was the hematocrit, and (1 − HCT) was the proportion of blood volume that was plasma. RMECFF and HCT reference values in women were 0.19 and 0.47, respectively (17,18).
For the 10 participants without bone disease, bone marrow activity was also estimated using SUVblood and Equation 5 at S0, S1, and S2. Statistical analysis was then performed to test for significant differences in the 28 paired datasets of  estimated from the blood pool using Equation 5 versus directly measured from the vertebral body using Equation 4.
estimated from the blood pool using Equation 5 versus directly measured from the vertebral body using Equation 4.
The fraction of activity retained within the whole body,  , was determined by substituting reference organ mass in Equation 3 with the reference body weight, 58,000 g, which reduced the calculation to…
, was determined by substituting reference organ mass in Equation 3 with the reference body weight, 58,000 g, which reduced the calculation to…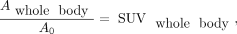 Eq. 6
Eq. 6
where SUVwhole body was the SUVmean within a VOI around all parts of the body within the image. In Equation 6, whole-body activity, including arms and legs outside the image, was estimated by scaling the mean activity sampled by the VOI to the total mass of the participant.
[18F]FDHT activity was removed from the body only through radioactive decay and urinary excretion during the time between radiopharmaceutical administration and PET/CT imaging. Removal of [18F]FDHT from the body by urinary excretion was modeled using a monoexponential decay function,  . Therefore, the biologic half-life of [18F]FDHT, Tbiol, was calculated as…
. Therefore, the biologic half-life of [18F]FDHT, Tbiol, was calculated as… Eq. 7
Eq. 7
where  was calculated using Equation 6, and Δt was the time between radiopharmaceutical administration and imaging.
was calculated using Equation 6, and Δt was the time between radiopharmaceutical administration and imaging.
The time-integrated activity coefficient in an organ,  , represents the cumulative number of 18F radioactive decays occurring per unit activity of [18F]FDHT during the time the activity remains in the organ. For this study, the
, represents the cumulative number of 18F radioactive decays occurring per unit activity of [18F]FDHT during the time the activity remains in the organ. For this study, the  values for the cerebellum, left ventricle heart blood pool, kidneys, liver, lungs, and spleen were calculated as…
values for the cerebellum, left ventricle heart blood pool, kidneys, liver, lungs, and spleen were calculated as… Eq. 8
Eq. 8
conservatively estimating monoexponential clearance of activity by radioactive decay only, where Tphy was the half-life of physical radioactive decay for 18F and  was calculated using Equation 3.
was calculated using Equation 3.
Time-integrated activity coefficient in the red bone marrow, 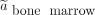 , was calculated using Equation 8 with
, was calculated using Equation 8 with  , where
, where 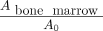 was derived using Equation 4 for the 10 participants without bone disease and Equation 5 for the participant with bone disease.
was derived using Equation 4 for the 10 participants without bone disease and Equation 5 for the participant with bone disease.
The time-integrated activity coefficient of urinary bladder contents,  , was calculated using the dynamic bladder tool in the OLINDA/EXM software (19) with a urinary bladder voiding time of 2 h. For the dynamic bladder model, Tbiol values from Equation 7 were the rate constant for urinary elimination, and the fraction of activity excreted via the urinary bladder was estimated as the fraction of activity not in the liver at the time of imaging, 1 − Aliver.
, was calculated using the dynamic bladder tool in the OLINDA/EXM software (19) with a urinary bladder voiding time of 2 h. For the dynamic bladder model, Tbiol values from Equation 7 were the rate constant for urinary elimination, and the fraction of activity excreted via the urinary bladder was estimated as the fraction of activity not in the liver at the time of imaging, 1 − Aliver.
The time-integrated activity coefficient in the remaining body tissues and organs was calculated as… Eq. 9
Eq. 9
where  was the sum of the fractions of activity calculated using Equation 3 in cerebellum, left ventricle chamber contents, kidneys, liver, lungs, spleen, and Equation 4 or Equation 5 in red bone marrow, and
was the sum of the fractions of activity calculated using Equation 3 in cerebellum, left ventricle chamber contents, kidneys, liver, lungs, spleen, and Equation 4 or Equation 5 in red bone marrow, and  for urinary bladder contents had been calculated by the dynamic bladder model.
for urinary bladder contents had been calculated by the dynamic bladder model.
RESULTS
[18F]FDHT Biodistribution
Eleven women, with a median age 59 y (range, 47–73 y), were enrolled in the [18F]FDHT PET/CT imaging substudy. Nine participants were imaged at all 3 time points, whereas 2 participants were imaged only at S0 and S1. PET/CT images in Figure 1 show the typical biodistribution of [18F]FDHT, and Table 1 presents averages of SUVorgan measurements in the 11 participants.
Biodistribution in typical female participant at 52 ± 6 min after administration of [18F]FDHT. Figure shows PET/CT images of participant with breast cancer imaged at S0, S1, and S2. At top are maximum-intensity projection images, and at bottom are coronal PET/CT images at level of gallbladder and bile duct.
Intersubject Averages of SUVorgan Measurements from [18F]FDHT PET/CT Images in 11 Women with Breast Cancer at S0, S1, and S2
As shown in Figure 1, blood-pool activity within the circulatory system remained higher than in surrounding muscle at 52 ± 6 min after administration of [18F]FDHT. Uptake was highest within the liver, spleen, and kidneys and in the gallbladder and urinary bladder, indicating gastrointestinal and urinary excretion of the radiopharmaceutical.
PET/CT images showed no changes in the visual appearance of the [18F]FDHT biodistribution at S1 and S2, compared with S0. However, SUVorgan measurements revealed a statistically significant decrease in liver uptake from S0 to S1 and S2 (linear mixed model, P < 0.05), as shown in Figure 2 and Table 1. A statistically significant decrease in SUV was also found for the aorta blood pool at S1 (linear mixed model, P < 0.05). Other than liver and aorta blood pool, no other source organs or regions showed significant changes in SUVorgan measurements at S1 or S2 compared with S0 (linear mixed model, P ≥ 0.05).
[18F]FDHT SUVorgan measurements in liver of women with breast cancer at S0, S1, and S2. (Left) Spaghetti plot showing changes in measurements in individuals across all time points. (Right) Box plot showing median, interquartile range, and mean (+) of measurements over the 11 participants at each time point. Linear mixed model analysis found statistically significant decrease in uptake in individual participants at S1 compared with S0 (P < 0.05) and no statistically significant change between S1 and S2 (P = 0.87). pt = patient.
Two participants had a prior history of cholecystectomy. Of the 9 participants with gallbladders, gastrointestinal accumulation of radioactivity was seen in the gallbladder and bile ducts at one or more of the imaging time points for 8 participants, whereas 5 participants showed gallbladder uptake at all 3 time points. Gastrointestinal excretion of activity into the large intestines was not yet observed at the time of imaging.
Urinary excretion was seen as activity in the renal pelvis draining into the ureters, and accumulation in the urinary bladder at the time of imaging, for all participants at all 3 time points. Participants were instructed to empty their urinary bladder before the PET/CT imaging. However, the amount of activity remaining within the urinary bladder in the images was visibly much greater than in the blood pool and varied among participants.
Dosimetry
There was no significant difference among the 28 paired data points of the fraction of activity in the red marrow, estimated from aorta blood-pool VOIs and Equation 5 versus lumbar spine VOIs and Equation 4 (2-tailed paired t test, P = 0.6).
Table 1 shows averages of the SUVorgan measurements in source organs and regions from the [18F]FDHT PET/CT images at each of the 3 time points. Time-integrated activity coefficients derived from these SUVorgan measurements are in the supplemental materials to this article (available at http://jnmt.snmjournals.org). Table 2 shows the resulting absorbed doses to target organs and the effective doses calculated at S0. The results for S1 and S2 are also in the supplemental materials.
[18F]FDHT Radiation Dosimetry in Women with Breast Cancer at S0: Absorbed Dose in Target Organ at S0
Absorbed doses calculated for the liver showed decreases at S1 and S2 compared with S0 that were found to be statistically significant (linear mixed model, P < 0.05). There were also statistically significant decreases in absorbed dose at S1 and S2 (linear mixed model, P < 0.05) for the gallbladder wall, stomach, pancreas, and adrenals, which are located very close to the liver. Absorbed doses calculated for breast tissue, heart wall, and total body showed small but statistically significant decreases at S1 only (linear mixed model, P < 0.05). However, the absorbed dose calculated for colon showed small but statistically significant increases at S1 only (linear mixed model, P < 0.05). No other organs or tissues showed statistically significant changes in absorbed doses compared with S0 (linear mixed model, P ≥ 0.05).
The urinary bladder remained the organ at risk at all 3 time points. Although the absorbed dose to the urinary bladder wall showed small increases at S1 and S2, the changes were not statistically significant (linear mixed model, P ≥ 0.05). Likewise, the effective doses calculated at S1 and S2 did not show statistically significant changes from S0 (linear mixed model, P ≥ 0.05).
The S0 values may be used as the reference values for absorbed dose in organs and the effective dose from [18F]FDHT in women, to eliminate the effect of SARM therapy on [18F]FDHT biodistribution. At S0, the effective dose was calculated as 0.020 ± 0.0005 mSv/MBq, and the urinary bladder was the organ at risk, with an average absorbed dose of 0.074 ± 0.011 mGy/MBq.
DISCUSSION
The liver plays a role in the metabolism of exogenous dihydrotestosterone androgen (20–22), and enobosarm is eliminated primarily through the hepatobiliary route (22). Our study found that [18F]FDHT SUV measured in liver significantly decreased over the course of SARM treatment from S0 to S1 and from S0 to S2, and the absorbed dose calculated to the liver also decreased. It is plausible that the change in uptake reflects the interaction of SARM therapy on androgen receptors in the liver; however, the complex mechanism by which SARM therapy affected the uptake in the liver and other normal tissues in not yet known.
Before the start of SARM therapy, the effective dose of [18F]FDHT was 0.020 ± 0.001 mSv/MBq in women with breast cancer, which is comparable to the 0.017 ± 0.002 mSv/MBq effective dose equivalent reported for men with prostate cancer (10), although the organ-weighting factors used for calculation of effective dose and effective dose equivalent are different. The organ at risk was the urinary bladder wall. The absorbed dose to the urinary bladder wall, 0.074 ± 0.011 mGy/MBq in women with breast cancer, was comparable to the published value of 0.087 ± 0.048 mGy/MBq in men with prostate cancer (10). The absorbed doses to the other organs in this study of women were generally 2 times higher than the previously published dosimetry in men with prostate cancer. A small increase of 10%–20% in organ doses is expected because of differences in organ masses and dose conversion S factors for the adult female versus adult male phantom. However, differences in phantom geometry and the S factors used in the dosimetry software versions can account for larger differences in organ dose calculations.
A limitation of this work is extrapolation of pharmacokinetics based on a single image acquired at approximately 1 h after administration of the [18F]FDHT. The biologic half-life due to urinary elimination of [18F]FDHT was then estimated using a 1-compartment model and the VOI measurements of the whole body at that time point. This calculation assumed that the fraction of administered activity that was not in the image had been eliminated through physical decay of the radioactivity and by urinary excretion only. This was a reasonable assumption, since gastrointestinal transit times in adults from the liver to the large intestines are on the order of 3–4 h. Urinary excretion was also assumed to have a single rate constant during the lifetime of the radiopharmaceutical within the body, which was a reasonable assumption for the short-lived 18F radioisotope.
For the participant with metastatic bone involvement, the bone marrow dosimetry was based on image-derived whole-blood activity concentrations with an assumption that there was no uptake in red blood cells and equilibrium between plasma and the marrow extracellular space. This assumption would hold for intact [18F]FDHT, which should behave like androgens and remain in plasma. However, 18F ions could be taken up by red blood cells. The presence of free 18F ions from defluorinated [18F]FDHT is documented in the literature in mouse and baboon prostate cancer models (1,2). Beattie et al. also showed that the activity concentration of [18F]FDHT metabolites in blood samples, from a group of 13 men with prostate cancer, exceeded the activity concentration of whole [18F]FDHT within the first 10 min after administration (11). Blood sampling studies in female populations are needed to derive more accurate red marrow–to–blood activity concentration ratios for [18F]FDHT, accounting for uptake of 18F ions in the blood cells and changes in the ratios over time.
Another limitation is that the number of participants included in the [18F]FDHT PET/CT imaging substudy was small and the study sample was limited to postmenopausal women with known breast cancer. As such, further research with larger sample sizes is needed to investigate if the dosimetry calculated in this sample accurately represents the average dose in women.
This article focused on normal-tissue dosimetry and not tumor dosimetry. Therefore, [18F]FDHT uptake in androgen receptor–positive tumors was not evaluated as separate source regions but was combined into the total-body measurements. Additionally, calculations of the time-integrated activity coefficients in this and other works use the assumption of instantaneous uptake within the organs immediately after administration and trapping with no washout of activity over time. This assumption overestimates the time-integrated activity coefficients. The Beattie et al. study of [18F]FDHT pharmacokinetics found that uptake within prostate tumors plateaued at 20 min (11). However, similar investigations of [18F]FDHT pharmacokinetics in organs are needed and will allow improved accuracy for future dosimetry studies.
CONCLUSION
Whole-body and organ radiation dosimetry from [18F]FDHT in women with breast cancer was comparable to the reported dose in men with prostate cancer. The effective dose in the women with breast cancer was 0.020 ± 0.0005 mSv/MBq. The urinary bladder was the organ at risk, with an absorbed dose of 0.074 ± 0.011 mGy/MBq to the urinary bladder wall.
DISCLOSURE
Research funding was provided in part by GTx, Inc. Heather Jacene has received honoraria from Munrol; research support to the institution from Siemens Healthcare, Inc., GTx, Inc., and Blue Earth Diagnostics; consulting fees from Advanced Accelerator Applications and Munrol; royalties from Cambridge Publishing; and NIH/NCI grant support not related to this work as coinvestigator (1R01CA235589-01A1). Beth Overmoyer has received clinical trial support from Genentech, Incyte, GTx, Inc., and Eisai. Annick Van den Abbeele has received a NCI National Comprehensive Cancer Center grant (Dana-Farber/Harvard Cancer Center 2 P30 CA006516-52; principle investigator, Laurie Glimcher) as the co–principle investigator in the Tumor Imaging Metrics Core; is an unpaid board member of the Centre for Probe Development and Commercialization (CPDC), Toronto, Canada; is an unpaid consultant to Fusion Pharmaceuticals and Bristol-Myers Squibb; has received travel expenses from Ipsen, ImaginAb, and CPDC to attend investigators’ or board meetings; and has received royalties from Thieme Publishers as the textbook coeditor of Case-Based Nuclear Medicine, second edition. Diane Young and Mayzie Johnston were employees of GTx, Inc. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the biodistribution of [18F]FDHT in women, and what are the doses to organs and the effective dose in women per unit activity of [18F]FDHT?
PERTINENT FINDINGS: This work investigated the biodistribution, organ dose, and effective dose of [18F]FDHT in women. Eleven women with metastatic breast cancer receiving selective androgen receptor modulation therapy on a therapeutic trial were enrolled in this prospective imaging substudy of [18F]FDHT.
IMPLICATIONS FOR PATIENT CARE: The biodistribution and dosimetry of [18F]FDHT indicate that it may be used in androgen receptor PET/CT imaging of women.
ACKNOWLEDGMENT
We thank Pat Zanzonico, PhD, for his expertise in reviewing and editing the manuscript.
Footnotes
Published online Jun. 14, 2023.
REFERENCES
- Received for publication January 3, 2022.
- Revision received March 21, 2023.










