Abstract
The process of bringing a new drug to market is complex and has recently necessitated a new drug-discovery paradigm for the pharmaceutical industry that is both more efficient and more economical. Key to this task has been the increasing use of nuclear medicine and molecular imaging to support drug discovery efforts by answering critical questions on the pathway to development and approval of a new therapeutic drug. Some of these questions include whether the new drug reaches its intended target in the body at sufficient levels to effectively treat or diagnose disease without unacceptable toxicity; how the drug is absorbed, metabolized, and excreted; and what the effective dose is in humans. To conduct the appropriate imaging studies to answer such questions, pharmaceutical companies are increasingly partnering with molecular imaging departments. Nuclear medicine technologists are critical to this process, as they perform scans to collect the qualitative and quantitative imaging data used to measure study endpoints. This article describes preclinical and clinical research trials and provides an overview of the different ways that radiopharmaceuticals are used to answer critical questions during therapeutic drug development.
For pharmaceutical companies, the process of discovering a new diagnostic or therapeutic drug and developing it for market is enormously complex (Fig. 1) and includes preclinical testing, investigational-new-drug (IND) applications, clinical trials, new-drug applications (NDAs), marketing approval from the U.S. Food and Drug Administration (FDA), and postmarketing studies. The goal is to bring safe and effective drugs to market as quickly as possible, but it can take up to 10 y to complete this drug development process, and the lifetime of a U.S. patent (during which pharmaceutical companies need to complete drug discovery, translation, and approval; establish the drug’s market share; recoup the research investment; and turn a profit) is only 20 y. Recent studies estimate the average cost of therapeutic drug development at $1.3 billion per drug, but it can be as high as $5.5 billion (1,2). This challenge is further exacerbated when promising drug candidates fail to meet prespecified endpoints in later-stage clinical trials after many years of expensive investment. Reimbursement from the Centers for Medicare and Medicaid Services and other insurance carriers also goes through a review process.
Drug development process. A(NDA) = abbreviated NDA; cGMP = current good manufacturing practice; CMS = Centers for Medicare and Medicaid Services.
These challenges have necessitated a new drug-development paradigm for the pharmaceutical industry that is both more efficient and more economical, and as a result, new technologies are being used to reduce both the costs and the risks associated with drug development. Molecular imaging and nuclear medicine are tools that play a key role in modern drug development because of their ability to address important questions at each step of the drug discovery process (2,3). For example, imaging studies costing a few tens to hundreds of thousands of dollars enable companies to reduce risk and more confidently make key go/no-go decisions about advancing drugs to clinical studies costing millions to billions of dollars (depending on size and scope). Such imaging studies require access to different radiopharmaceuticals (e.g., established radiotracers, new radiotracers, and radiolabeled drug candidates) and can be both preclinical and clinical. For example, early drug discovery efforts involve preclinical studies (e.g., in vitro autoradiography, in vivo animal imaging, and ex vivo biodistribution), whereas later drug development trials pivot to clinical studies on human volunteers (phase I, II, and III clinical trials conducted under approved IND applications). On completion of phase III studies, an NDA can be filed and, once approved, give the sponsor marketing authorization for the drug. Postmarketing phase IV trials may be required by the FDA for long-term surveillance of drug safety and efficacy.
In this primer, we describe regulatory and logistical considerations for nuclear medicine departments to participate in preclinical and clinical research, as well as an overview of the different ways that radiopharmaceuticals can be used to answer critical questions pertaining to therapeutic drug development (2,3). This paper is a companion to other articles in this series published to date (4).
TYPES OF IMAGING STUDIES CONDUCTED DURING DRUG DEVELOPMENT PROCESS
Nuclear medicine imaging (with PET and SPECT) enables industry and academic teams to answer questions central to new-drug development. PET and SPECT are increasingly used to answer questions during both preclinical in vitro (cells) and in vivo (animal models) studies and during clinical trials (human research subjects), including the following: Does a drug reach the tissue of interest in pharmacologically active concentrations? Does the drug go anywhere else in the body that could cause unwanted side effects? Does the molecule engage with the target (e.g., receptor or protein) of interest? What is the quantitative relationship between the extent of the drug’s interaction with the target and the dose administered to a patient (e.g., receptor occupancy)? Can the pharmacologic effects of a therapeutic dose be determined using an imaging biomarker (e.g., tumor shrinkage or 18F-FDG metabolism and uptake (3)), and how long do these effects persist? The answers to these questions are critical to translating a drug to initial clinical trials, as well as to advancing the drug through clinical trials (phases I–III), and imaging offers a powerful means for drug discovery teams to address such questions.
The questions concerning the distribution and target engagement of a drug can often be answered by labeling a drug molecule itself. For example, radiolabeling of a drug with a PET radionuclide, such as 11C, can be accomplished without altering the properties of the drug. Since it is estimated that about 20% of prescribed drugs and about 30% of leading blockbuster drugs contain a fluorine atom (5), in such cases the same can also be accomplished with 18F (6). The labeled drug can then be used in preclinical and clinical biodistribution studies.
PET imaging can also be used to quantify the pharmacologic effects of a therapeutic dose. Such imaging capitalizes on the concept of biologic markers (i.e., biomarkers). A biomarker is an objective measurement that is an indicator of a biologic process in a patient and can serve as an indicator of health. Classic biomarkers are based on laboratory tests (e.g., blood, urine, and tissue biopsy), whereas an imaging biomarker is a measurement of a biologic process, such as quantification of amyloid burden using amyloid PET. Frequently, these types of studies might use companion PET radiotracers rather than a radiolabeled drug. Before commencing such studies, it is necessary to confirm that a radiotracer is appropriate for that purpose. When validating a PET tracer, a test–retest experiment is usually conducted to measure the repeatability of the measurements and determine within-subject variability (7). The test–retest is particularly important if a PET radiotracer is to be used in studies involving multiple measurements on the same subject (e.g., receptor occupancy or before and after a therapeutic intervention).
If an imaging biomarker exists for a given condition, it can be used to diagnose disease and both predict and monitor patient response to experimental new therapeutics in clinical trials. Extending the latter concept further, imaging biomarkers can also be used as a surrogate endpoint in a clinical trial. A surrogate endpoint is defined by the FDA as “a clinical trial endpoint used as a substitute for a direct measure of how a patient feels, functions, or survives” (8). Imaging biomarkers and surrogate endpoints are recognized by the agency and have been widely used in the drug approval and licensure process (8,9). The main difference between an imaging biomarker and a surrogate endpoint is the level of validation. For an imaging biomarker to function as a surrogate endpoint, there must be clinical trials demonstrating the relationship between the imaging biomarker and the true clinical endpoint. Imaging biomarkers have been reviewed (10), and in the case of cancer trials, an imaging biomarker road map has been established (11). Examples of imaging biomarkers used as surrogate endpoints include amyloid imaging (12), assessment of tumor response (13–15), and 11C-raclopride PET for antipsychotic efficacy (16,17).
REGULATORY CONSIDERATIONS
Use of nuclear medicine imaging techniques to support drug development involves both preclinical and clinical studies. Such work must be conducted in compliance with applicable institutional, state, and federal regulations governing the use of radioactive material, as well as the rules and regulations describing responsible and ethical conduct in animal and human research. These various regulatory requirements are summarized briefly here and are covered in detail in other articles in this series (4).
Radioactive materials need to be handled under the auspices of approved radioactive materials licenses granted by the Nuclear Regulatory Commission or the local state government for agreement states. Such work must also be performed according to the as-low-as-reasonably-achievable principles, which involve making every reasonable effort to ensure that worker exposures to ionizing radiation are as low as practical.
In the United States, PET radiopharmaceuticals are prepared according to the principles of current good manufacturing practices outlined in the U.S. Pharmacopeia chapter <1823> (18) or chapter <825> (19) and in part 212 of title 21 of Code of Federal Regulations (CFR) (20). Other radiopharmaceuticals (e.g., radiotherapeutics) are often prepared according to requirements outlined in 21 CFR parts 210 (21) and 211 (22). The regulations on current good manufacturing practices cover types of facilities, cleanliness and maintenance of the facilities, laboratory controls, equipment, personnel, training, quality assurance, documentation about materials and processes, drug product controls, packaging and labeling requirements, complaint handling, and record keeping.
Preclinical (animal) studies need to be conducted under the purview of an Institutional Animal Care and Use Committee, and it is typical for pharmaceutical companies to also require collaborating sites to hold Association for Assessment and Accreditation of Laboratory Animal Care International certification. The use of animals to advance medicine and science when there are no nonanimal alternatives remains a critical part of drug development, and preclinical work should be conducted in accord with the highest scientific, humane, and ethical principles as laid out in the Guide for the Care and Use of Laboratory Animals (23).
Clinical studies can be conducted only after receiving both FDA approval (e.g., Radioactive Drug Research Committee [RDRC] or IND application) and institutional approval (e.g., Institutional Review Board). Regulations for using radiopharmaceuticals under RDRC approval are described in 21 CFR part 361, whereas the requirements for conducting research under an IND are laid out in 21 CFR part 312. For a detailed description, refer to a companion article in this series (4).
TYPES OF RESEARCH STUDIES
Preclinical Studies
Although nuclear medicine technologists work predominantly in clinical PET imaging, some may find themselves working in academic PET centers or pharmaceutical companies. In the latter instances, they will likely be involved in preclinical studies. Preclinical studies are intended to get earlier answers to many of the same questions that will ultimately be investigated clinically and often to make go/no-go decisions about costly clinical translation. There are 4 main types of preclinical protocols: cell studies, autoradiography experiments with postmortem tissue samples (in vitro or ex vivo), in vivo PET (or SPECT) imaging studies on living animals, and ex vivo biodistribution studies in which animals are euthanized after injection of the tracer, dissected, and their organs counted in a γ-counter to establish the biodistribution and dosimetry of the tracer.
Cell Studies
Cell uptake studies evaluate uptake of a new radiotracer in a cell expressing the drug target of interest. These studies are an economic starting point as they cost considerably less than animal studies. Cell uptake studies offer a preliminary indication of target engagement for a new radiotracer and also help researchers decide promising ones to advance to animal studies.
Binding Affinity Experiments
Binding studies quantify the binding characteristics of a new radiopharmaceutical, such as affinity for its target, which is expressed as the dissociation constant (KD). Lower KD values correspond to higher-affinity molecules. Such studies can be conducted using tissue pellets or autoradiography.
Autoradiography Experiments
Autoradiography is a technique in which tissues are incubated with a radiotracer (24) and then exposed to photographic film or phosphor imaging plates to visualize the location of the radiolabeled molecules. In vitro autoradiography studies use postmortem animal or human tissue samples (e.g., a histologic slice of brain or tumor) that have been previously harvested. The radiolabeled molecule is incubated with postmortem tissue samples, giving the molecule time to bind to its target (e.g., receptor or protein). The samples are then washed and exposed to film or plates. In vitro autoradiography does not account for the in vivo environment (e.g., metabolism). If such information is needed, ex vivo autoradiography studies can be undertaken. The radiotracer is first administered intravenously, and after some time point, the animals are euthanized (with or without in vivo PET imaging first) and organs and tissues of interest are harvested for autoradiography. The postmortem tissue samples are washed and exposed to film or plates analogously to in vitro autoradiography.
Autoradiography has been used to quantify and localize drugs in organs, tissues, and cells for decades. The data can be used to determine KD for a radiotracer, and target engagement of a therapeutic drug can be confirmed by observing a reduction in the signal of the specific radiotracer in the presence of a therapeutic dose (either dosed to the animal [ex vivo autoradiography] or added to the incubation solution [in vitro autoradiography]). Alternatively, the technique can be combined with immunohistochemistry on an adjacent slice of tissue, and colocalization of the autoradiography signal with the fluorescence of target-specific antibodies can be observed. Such datasets are critical in validating a new radiotracer and deciding whether to advance to preclinical and eventual clinical in vivo imaging studies.
In Vivo PET (or SPECT) Imaging Studies
In vivo imaging study logistics closely resemble human studies in that doses, imaging protocols, timing, intravenous catheter placement, interventions, attenuation correction, reconstruction, and radiation safety all have to be accounted for and planned in the same way. An additional consideration with animal work is that, unlike human subjects, animals need to be anesthetized for the duration of the study. Depending on the mechanism of action of a drug, the choice of anesthesia can affect the PET data and may need to be accounted for in study design (25). For preclinical PET imaging, small mice typically receive no more than 9.25 MBq of radiotracer, whereas larger animals receive higher doses depending on weight (e.g., rodents receive ≤37 MBq and primates receive ≤185 MBq). PET scans can be dynamic, and typical scan lengths are 60 min for a 11C-labeled tracer or up to 120 min for an 18F-labeled tracer. Static scans, which could be 5–20 min, can also be acquired at one or more time points after administration of the radiotracer. Baseline scans will give pharmacokinetic and distribution information on a given tracer or labeled drug candidate, whereas intervention studies (e.g., with a dose of therapeutic) can be used to answer questions about specific binding, target engagement, and receptor occupancy at a given dose, among others. In some studies, the animal may be euthanized immediately after the imaging study and organs harvested for further evaluation using autoradiography. Figure 2 shows a baseline nonhuman-primate PET scan obtained with 18F-AH114726, a new radiotracer targeting the γ-aminobutyric acid type A receptor (26). The scan reveals high uptake in the cortical region, which is known to have high expression of γ-aminobutyric acid type A receptors. To confirm selectivity of the new compound for the target, the scan was repeated in the presence of a 1 mg/kg dose of flumazenil, a known selective γ-aminobutyric acid type A receptor antagonist. The repeated scan confirmed target engagement and selectivity of AH114726, as complete displacement of the radiotracer was apparent on dosing with flumazenil.
Representative coronal small-animal PET images of control animal imaged with 18F-AH114726 at baseline and after displacement with 1 mg/kg dose of flumazenil. (Reprinted with permission of (26).)
Biodistribution Studies
Ex vivo biodistribution studies are operationally more complex than in vivo imaging, as they involve euthanizing animals at a predetermined time point after dosing the radiotracer, dissecting them, and counting the individual organs in a γ-counter. Such studies provide comprehensive biodistribution data (percentage injected dose per gram of tissue) that can be input into software programs such as OLINDA (27) to generate the human dosimetry estimates that need to be included in any IND filings supporting translation of PET radiotracers into clinical studies.
Clinical Studies
Clinical studies, using PET, most frequently apply established radiotracers that are either FDA-approved or investigational. Investigational radiotracers can be used under the approval of an institutional RDRC committee or an FDA-approved IND, depending on the intended application (4,28). Approved radiotracers are used under the auspices of either an NDA or, in the case of generic drugs, an abbreviated NDA. The different phases of clinical trials are summarized in Table 1 and described in detail in a previous article in this series (4).
Overview of Clinical Trial Process
HOW RADIOPHARMACEUTICALS CAN BE DEPLOYED IN RESEARCH
Several types of radiopharmaceuticals are used in imaging collaborations with pharmaceutical companies (Table 2). They include use of FDA-approved products as tracers or biomarkers to study aspects of disease, investigational agents to interrogate biologic systems and functions, investigational imaging or therapeutic radiopharmaceuticals being developed for FDA approval, and radiolabeled drug compounds to study biodistribution and receptor occupancy. The selection of an appropriate imaging agent depends on the study in question and on local availability. For example, different imaging agents might be used at various sites in the same clinical trial, depending on availability from nearby commercial nuclear pharmacies.
Radiopharmaceuticals Used in Clinical Trials
FDA-Approved Imaging Radiopharmaceuticals for Diagnosis, Staging, and Monitoring of Therapy
Approved radiopharmaceuticals can be used for diagnosis of disease, staging, and monitoring of response to therapy (experimental or approved). Importantly, if patients respond to therapy they continue receiving the same treatment, whereas if they do not respond they can be switched to alternate therapies rapidly, saving both time and money and reducing the duration of unnecessary side effects. Pioneering work was conducted by the team of Van den Abbeele at the Dana-Farber Cancer Institute to diagnose and treat gastrointestinal stromal tumors (GISTs) (28). 18F-FDG PET can be used to diagnose, localize, and stage GISTs and to monitor response to imatinib (Gleevec; Novartis) therapy (Fig. 3). The functional information on tumor metabolism of glucose from serial 18F-FDG scans can be used to detect both short-term and long-term tumor responses that may not be obvious with CT. The team noted that 18F-FDG PET responses were apparent even 24 h after the first dose of imatinib. Significant changes in 18F-FDG uptake (>25% decrease in SUVmax relative to baseline) were apparent within 1 mo of starting imatinib therapy in all GIST patients who responded. Any lack of response was quickly apparent on the PET scans, such that treatment could be changed to sunitinib (Sutent; Pfizer), which is a second drug approved for treatment of GIST and to which non–imatinib-responding patients may be more sensitive.
18F-FDG PET maximum-intensity projections (A–C), axial PET images (D–F), and axial CT images (G–I) through pelvis in patient with metastatic GIST. Normal physiologic 18F-FDG uptake is seen in urinary collecting system in both kidneys (kd), in myocardium (my), and in bladder (bl). (A) Intense 18F-FDG uptake is seen in left lower pelvis and contiguous to right proximal ureter (TU) at baseline before imatinib therapy, consistent with metastatic GIST. (B) Resolution of abnormal 18F-FDG uptake is noted in both tumor masses as early as 1 wk after treatment. (C) Continuous metabolic response to imatinib is seen in this patient 2 mo after initiation of therapy despite presence of residual mass on CT. (Reprinted with permission of (29).)
Use of FDA-Approved Imaging Agents as Biomarkers or Surrogate Endpoints in Clinical Trials
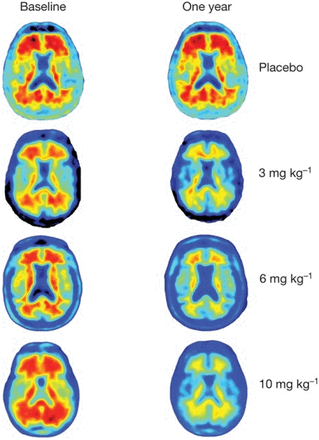
A recent example of using PET radiotracers to support therapeutic trials applied amyloid imaging (18F-florbetapir [Amyvid; Eli Lilly], 18F-flutemetamol [Vizamyl; GE Healthcare], and 18F-florbetaben [Neuraceq; Life Molecular Imaging]) and tau PET (18F-flortaucipir [Tauvid; Eli Lilly] and MK6240) to support development of Alzheimer disease therapeutics such as aducanumab. Such therapeutic agents are recombinant human monoclonal antibodies that bind aggregated types of β-amyloid that form the hallmark amyloid plaques in Alzheimer disease. PET imaging played a crucial role in confirming the initial eligibility of a given patient to participate in a clinical trial and in monitoring the subsequent response to therapy. In clinical trials of aducanumab, amyloid PET revealed decreases in β-amyloid neuritic plaque accumulation on treatment (Fig. 4) (29).
18F-florbetapir (Amyvid) amyloid PET images at baseline and 1 y after aducanumab treatment, showing amyloid plaque reduction after different doses of aducanumab but not placebo. (Reprinted with permission of (30).)
Investigational Agents for Research Use Under RDRC or IND
Imaging agents can be used for research applications under the approval of an institutional RDRC (30) or under an approved IND (31). To use a radiotracer according to the RDRC mechanism, the following provisions must be met: the research must be considered basic science research and be done for the purpose of advancing scientific knowledge; the research study must be authorized by an FDA-approved RDRC; the pharmacologic dose of the radioactive drug must be known not to cause any clinically detectable pharmacologic effect on humans; and the radiation dose to be administered must be justified by the quality of the study and the information it seeks to obtain. If these provisions cannot be met, an approved IND application is required for the agent before any human research can be conducted (32,33). Academic medical centers frequently have many established radiotracers available for use in RDRC protocols and likely also hold IND approvals for several additional radiotracers. Additional research protocols can be added to INDs by submission of amendments to the FDA. After a radiotracer has been used in humans under an IND, it can be advanced to additional clinical trials with a goal of commercialization. Alternatively, if the pharmacologic dose did not cause any clinically detectable pharmacologic effects, it can subsequently be transitioned to RDRC studies for additional research, assuming the other criteria are met.
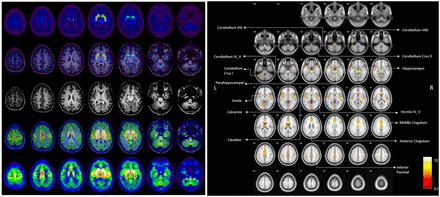
The transition from RDRC to IND can be illustrated by 18F-(–)5-fluoroethoxybenzovesamicol (18F-FEOBV), a PET radiotracer that was developed at the University of Michigan and is based on a vesamicol analog used to image the vesicular acetylcholine transporter (34). After preclinical development along the lines described in this article, the team wrote an IND and received approval from the FDA to proceed with first-in-humans studies. Whole-body 18F-FEOBV scans were initially conducted on 3 healthy volunteers. Seven additional subjects underwent dynamic brain imaging (Fig. 5), and kinetic modeling revealed agreement between reference tissue modeling and late single-scan imaging. This study allowed quantification of human dosimetry, indicating that more than 400 MBq could be administered without exceeding radiation dose limits. No pharmacologic or physiologic changes were observed after intravenous administration of no more than 1.3 μg of 18F-FEOBV. This information subsequently enabled use of 18F-FEOBV under RDRC approval (and related mechanisms in other countries) by other research teams in the United States and elsewhere (35–37). For example, Kanel et al. recently used 18F-FEOBV to investigate age-related declines in regional cholinergic neuron terminal density (Fig. 5) (38).
Investigational Imaging and Therapeutic Agents Being Developed for Commercialization
Development of new radiopharmaceuticals for commercialization follows the process illustrated in Figure 1. Since companies do not have access to research subjects, clinical trials are usually conducted in collaboration with academic medical centers. After preclinical work, an IND is obtained to enable clinical trials with new agents to prove safety and efficacy. High-profile examples are the theranostic agents targeting prostate-specific membrane antigen (PSMA) for imaging and treatment of prostate cancer. In the case of 68Ga-PSMA-11, after pioneering work from Heidelberg, an NDA for 68Ga-PSMA-11 was obtained by UCLA and the University of California San Francisco through an academic partnership (39). They conducted a phase III trial, and separate NDAs for each institution were approved by the FDA. Subsequent approvals for kits to produce 68Ga-PSMA-11 have been obtained by Telix and Novartis, and the agent has been used to image thousands of patients (40).
Concurrently with these efforts, 177Lu-PSMA-617 was developed for radiotherapy of prostate cancer. PSMA PET is used to confirm patient eligibility and to monitor response to therapy. Phase 2 studies showed promising results (Fig. 6) (41), and the rights for development were acquired by Endocyte (later purchased by Novartis). In the large, international, multicenter phase 3 VISION trial, 831 patients underwent randomization. VISION provided evidence for significantly improved progression-free and overall survival in late-stage prostate cancer patients who were treated with 177Lu-PSMA-617 (compared with the standard of care) (42,43). FDA approval for 177Lu-PSMA-617 was granted earlier this year.
68Ga-PSMA-11 PET before and after treatment with 177Lu-PSMA-617. (Reprinted from (42).)
New Radiotracers Developed to Support Therapeutic Trials
If radiotracers do not exist for a given therapeutic target, it is necessary to develop the appropriate companion diagnostic when PET studies are needed. One seminal report on the use of PET imaging to guide therapeutic development was Merck’s work with Emend (aprepitant), a neurokinin 1 receptor antagonist being developed both for treatment of chemotherapy-induced nausea and as an antidepressant (44). Instead of labeling the drug itself, Merck developed a companion tracer, 18F-SPARQ (Substance P Antagonist Receptor Quantifier), and used it to determine the receptor occupancy achieved by different doses of Emend in healthy humans. Importantly, greater than 95% receptor occupancy was found at the proposed dose (Fig. 7), indicating the correct dose selection. Merck knew that this dose was effective for managing nausea caused by chemotherapy, moved forward with the antinausea indication, and gained marketing approval from the FDA. In contrast, Merck also knew that the study dose of Emend had no antidepressant effects, and the PET study revealed that a larger dose would not increase receptor occupancy. As such, the results of this study (likely costing tens of thousands of dollars) allowed Merck to cancel development of Emend for depression and save millions of dollars on what would have been a futile phase III trial (44).
(Left) 18F-SPARQ PET images of subject who received 100 mg of aprepitant: predosing (top row) and postdosing (bottom row) are shown, with estimated receptor occupancy of 94%. (Right) Relationship between plasma concentration of aprepitant and receptor occupancy. NK1 = neurokinin-1. (Reprinted with permission of (45).)
Radiolabeled Drugs or Drug Candidates
Important questions concerning a drug can be answered by labeling the drug molecule itself. Clinical imaging studies using labeled drugs allow drug developers to obtain information about biodistribution and target engagement. This information can be used to confirm patient eligibility for a given treatment, as well as to predict response. This approach holds particular promise for anticancer drugs. In a given malignancy, certain chemotherapeutics will be used on the basis of demonstrated clinical efficacy (e.g., tumor response or improved survival). A one-size-fits-all approach has been used in which patients are started on a standard regimen for their cancer. However, failure of a given chemotherapeutic occurs in certain patients because many cancers do not respond consistently. Therefore, nonresponders are often subjected to the psychologic burden of chemotherapy along with frequent associated toxicities without gaining any benefit. In this age of personalized medicine, a new era of chemotherapy that abandons this one-size-fits-all approach is needed. Molecular imaging has an important role to play in this paradigm shift.
Proof of concept for docetaxel has been demonstrated by van der Veldt et al. at VU University Medical Center in Amsterdam. This cytotoxic drug is a taxane that was initially approved for treatment of anthracycline-refractory metastatic breast cancer and has subsequently been approved for treatment of several other cancers (e.g., metastatic prostate cancer, head and neck cancer, and non–small cell lung cancer). However, cancers often do not respond consistently to chemotherapeutics. Docetaxel failure in some patients means that they experience associated toxicities without any benefit. To explore a more personalized approach to deciding which patients would benefit from docetaxel therapy, the team prepared 11C-docetaxel (45). Using PET imaging in conjunction with microdoses of 11C-docetaxel (to eliminate toxicity concerns), the team was able to quantify tumor uptake of the labeled drug across patients. This work showed that 11C-docetaxel PET can be used to predict tumor uptake of the drug during subsequent docetaxel therapy, and the team also demonstrated that high tumor uptake of 11C-docetaxel was related to improved tumor response after treatment.
Going forward, PET with radiolabeled drugs will enable straightforward confirmation of target engagement in humans and, in turn, predict treatment outcome in advance. The work with 11C-docetaxel also suggests that radiolabeled drugs can help to reveal underlying mechanisms of treatment failure in subpopulations of patients (e.g., poor accumulation in a tumor or poor target engagement).
CONCLUSION
The power of nuclear medicine and molecular imaging to provide key information about new therapeutic candidates has become an integral part of the modern drug development paradigm. Preclinical studies can allow early go/no-go decisions about whether to advance a new drug to clinical trials. After clinical translation, imaging can be used to enrich clinical trials, predict response to therapy, monitor response, and obtain valuable information about target engagement and dosing. In this way, it is expected that nuclear medicine and molecular imaging using radiotracers or radiolabeled drugs will continue to provide insights during drug development. When combined with other emerging technologies, this approach will usher in a new era of personalized medicine in which rational treatment choices tailored to individual patients to improve outcomes will replace a one-size-fits-all approach to disease management.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We thank LisaAnn Trembath from Avid Radiopharmaceuticals for her subject matter expertise and input on continuing education questions.
Footnotes
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than December 2025. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive 1.0 CEH (Continuing Education Hour) credit. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
Published online Jun. 14, 2022.
REFERENCES
- Received for publication May 4, 2022.
- Revision received June 11, 2022.