Abstract
Spirometric gating devices (SGDs) can measure the respiratory signal with high temporal resolution and accuracy. The primary objective of this study was to assess the feasibility and tolerance of a gated lung PET/CT acquisition using an SGD. The secondary objective was to compare the technical quality, accuracy, and interoperability of the SGD with that of a standard respiratory gating device, Real-Time Position Management (RPM), based on measurement of vertical thoracoabdominal displacement. Methods: A prospective phase I monocentric clinical study was performed on patients undergoing 18F-FDG PET/CT for assessment of a solitary lung nodule, staging of lung malignancy, or planning of radiotherapy. After whole-body PET/CT, a centered gated acquisition of both PET and CT was simultaneously obtained with the SGD and RPM during normal breathing. Results: Of the 46 patients who were included, 6 were prematurely excluded (1 because of hyperglycemia and 5 because of distant metastases revealed by whole-body PET/CT, leading to an unjustified extra gated acquisition). No serious adverse events were observed. Of the 40 remaining patients, the gated acquisition was prematurely stopped in 1 patient because of mask discomfort (2.5%; confidence interval [CI], 0.1%–13.2%). This event was considered patient tolerance failure. The SGD generated accurately gated PET/CT images, with more than 95% of the breathing cycle detected and high temporal resolution, in 34 of the 39 patients (87.2%; 95% CI, 60.0%–100.0%) and failed to generate a biologic tumor volume in 1 of 21 patients with increased 18F-FDG uptake (4.8%; 95% CI, 0.1%–26.5%). The quality and accuracy of respiratory signal detection and synchronization were significantly better than those obtained with RPM (P < 0.05). Conclusion: This trial supports the use of an SGD for gated lung PET/CT because of its high patient tolerance and accuracy. Although this technique seems to technically outperform RPM for gated PET/CT, further assessment of its superiority and the clinical benefit is warranted. We believe that this technique could be used as a gold standard to develop innovative approaches to eliminate respiration-induced blurring artifacts.
Imaging with 18F-FDG PET/CT is a pivotal modality for lung malignancy diagnosis and staging, because of its high accuracy (1,2). However, respiratory motion (3) can result in improper evaluation of radiotracer uptake (4,5), overestimation and deformation of tumor volume (6), misregistration of PET/CT data (7), and decreased accuracy of tumor localization (8). Currently, no consensus exists on a correction method for breathing motion during PET/CT acquisitions. Respiratory gating has been previously investigated in CT and PET/CT to reduce breathing-induced artifacts, based on spirometry (9–11) or on abdominal height measurement with Real-Time Position Management (RPM) (Varian) (12) or the Anzai Belt (13) (AZ-733V; Anzai Medical Co.).
Spirometric measures based on internal lung air content produce a stronger, more reproducible relationship and a shorter time lag than measures based on abdominal movement (10). Respiratory volumes correlate better with tumor motion than with abdominal motion (14). These quantitative analyses support the use of spirometry for gated CT scans. Although the implementation of spirometry can be challenging because of baseline drift (15), solutions to address that problem have been proposed (16–18).
A comparison study revealed a good correlation between RPM and spirometry (10,19) during PET acquisition. Software-based systems have recently been implemented in various forms (20). These data-driven gating methods use raw PET data to estimate the respiratory signal, yet they are still under development and are not currently applicable to CT. Currently, the only commercially available solutions are based on external devices. With these, failure rates of 15%–30% have been reported for respiratory gating examinations (21), caused by patient movement or incomplete data due to technical issues.
In the present study, we investigated a real-time pneumotachograph-based spirometric gating device (SGD). It measures the physiologic breathing signal by processing trigger signals with high temporal accuracy (22). On a moving PET phantom with 25-mm amplitude, it was able to reduce the smearing effect by increasing the maximal uptake (≤39.0%) and reducing the spreading of tumor-simulating spheres (≤2.7 times lower). It was able to detect, with high accuracy, breathing signals for a wide flow range (1.1–12.96 L min−1).
At present, the superior accuracy of SGDs over RPM demonstrated on phantoms and healthy volunteers has to be challenged under clinical conditions. A full-facial mask may cause patient discomfort, a claustrophobic sensation, and difficulties in eliminating airway secretions (23,24). Moreover, it could lead to more irregular respiratory movements in both amplitude and frequency, possibly hampering the binning accuracy of SGDs.
We performed a prospective phase 1 feasibility and tolerance study of an SGD for PET and CT of the lung, in comparison to standard RPM.
MATERIALS AND METHODS
Patient Selection
Because of the suspected clinical benefit of SGDs on respiratory gating, this study included mostly those patients who had been referred for assessment of small or medium solitary pulmonary nodules. The inclusion and exclusion criteria are presented in Table 1.
Inclusion and Exclusion Criteria
Endpoints
The primary endpoint was to determine the feasibility and failure rate of the entire gating process using the SGD. Three criteria needed to be fulfilled for the SGD process to qualify as successful: patient must tolerate the mask during training and acquisition; percentage of respiratory cycles in which signal synchronization was successful (dCycle) must be superior to 95%; and the lag between inhalation peak detection (maximum of the respiratory cycle) and trigger (dt) must be lower than 100 ms. Accuracy aims at assessing the reproducibility of the time-binning process. The number of breathing cycles and the maximum inspiratory amplitude of each cycle were retrospectively processed with in-house software and, if necessary, adjusted by a medical physicist.
The secondary endpoints were to compare the SGD with RPM in terms of quality, accuracy, and baseline drift (a drifting of the signal away from the nominal position of 0 between the beginning and end of the breathing cycle). For SGDs, baseline drift is often due to leakage of air in the system during each cycle or to a change in air temperature during inspiration and expiration. For RPM, baseline drift can be due to movement of the RPM box (i.e., table movement that is not well accounted for) or to a slackening or change in contraction of the abdominal muscles. Interoperability was defined as the ability to produce images for biologic tumor volume (BTV), and calculation of the SUVmax, using commercially available software on each bin.
Institutional Review Board Approval
This prospective phase I clinical study was approved by the French Health Authority (Agence Nationale de Sécurité du Médicament et des Produits de Santé; registration number UEC/DA2012) and the Ethics Committee (Comité de Protection des Personnes du Sud-Ouest et Outre-Mer III). All study participants received and signed an informed consent form. This study was registered in the ClinicalTrials.gov database (NCT01720186).
Standard PET/CT and Respiratory Gating
Preparation and Acquisition Protocol
After signing the informed consent form, the patients received a short physical examination and were trained to breathe with the SGD facial mask (placed over the mouth and nose) for up to 10 min.
All patients fasted for at least 6 h before the study. When glycemia was below 190 mg/dL, each patient received intravenous 18F-FDG (Cyclopharma) with an activity of 3.2 MBq/kg. After 60 min of rest and hydration, whole-body (skull base to mid thigh) standard PET/CT (Discovery ST; GE Healthcare) was performed, using 6–8 sequential bed positions (2 min 30 s per bed position, 70-cm field of view, and a 128 × 128 matrix, with CT parameters 120 kV, 30 mA, and a 256 × 256 matrix), interpreted by a nuclear medicine physician. Patients were excluded from the study if they had distant metastases because in such cases respiratory gating was clinically useless with respect to the patient’s condition. Such occurrences were not considered an SGD failure.
A second gated PET/CT acquisition centered over the pulmonary region of interest was performed with simultaneous use of both the SGD full facial mask and the RPM box on the abdomen. A flexible tube was plugged into the mask to connect to the synchronization system. Patients were asked to breathe normally. Figure 1 illustrates patient positioning during the SGD PET/CT acquisition. The gated PET acquisition took 12 min and was followed by the gated CT acquisition. We monitored blood oxygen saturation (percentage SaO2, with a finger pulse oximeter), heart pulse rate and blood pressure at baseline, during training and during the entire gated acquisition, under the supervision of the nuclear medicine physician. If the acquisition had to be stopped because of patient discomfort or a significant alteration in vital signs, the acquisition was considered an SGD failure.
SGD PET/CT setup. (A) SGD device. (B) Patient positioning by computer-assisted design. (C) SGD device position on PET/CT camera with screen monitoring.
Respiratory Gating Analysis
For the SGD, triggers were emitted toward the PET acquisition system at every inhalation peak generated by breath flow (22). For RPM, peak detection was based on abdominal movement. Respiratory signals issued by the SGD and RPM were simultaneously acquired generating 2 respiratory cycles as 2 stamp binning files (.vxd). PET data at reference points could provide different sonograms according to their acquisition time. Reference points for gating devices (RPM or the SGD) corresponded to inhalation peaks for each breathing cycle.
Two reconstructed image data sets (SGD and RPM-gated) were obtained using AdvantageSim 4D software on an Advantage (GE Healthcare) Windows (Microsoft) workstation. If BTV or SUVmax could be determined on the ungated images but not on the SGD images, the acquisition was also considered an SGD failure.
Statistical Analysis
The primary endpoint was feasibility, expressed as failure rate (absolute number and percentage, with corresponding 95% confidence interval [CI] [binomial exact]). All statistical analyses were performed using Stata software (version 13.1; StataCorp).
RESULTS
Patient Population and Characteristics
Forty-six patients were included in this study. Their baseline characteristics and medical history are presented in Table 2 and reflect the distribution of the standard population in our daily clinical setting. The PET indications are presented in Table 3.
Patient Characteristics (n = 46)
PET/CT Indications of Study Population
SGD Training, Patient Study Exclusion, and Feasibility of Gated PET/CT Acquisition
The SGD training phase lasted a median of 5 min (range, 2–6 min) and was successful, with none of the 46 patients experiencing any difficulty. Six patients were prematurely excluded after the training and waiting period (Fig. 2), because of unexpected metastases found on the standard whole-body PET/CT acquisition (5 patients) or a glycemia level higher than 1.9 g/L (1 patient).
Patient and data flowchart. dCycle (cutoff set at 95%) and dt (cutoff set at 100 ms) assess reproducibility of time binning operated by SGD and RPM devices. Time lag represents difference between synchronization signals sent to PET and maximum inspiratory amplitude for each cycle detected by device (RPM or SGD). It is a parameter for accuracy of detection of maximal inspiration amplitude and time needed for system to send this information to PET.
One of 40 patients (2.5%; 95% CI, 0.06%–13.16%) had a tolerance failure because of mask discomfort during the gated acquisition. The SGD acquisition was thus considered a failure. Blood oxygen saturation, heart pulse rate and blood pressure did not change between the baseline period, the gating training period and the gated PET/CT acquisition.
SGD and RPM Respiratory Gating Analysis
The median quality at which the SGD detected the inhalation peak was 100.0% (95% CI, 86.3%–102.1%), with a time lag of 25 ms (95% CI, 5.7–146.4 ms) and an end-of-expiration baseline drift of 8.6% (95% CI, 2.5%–53.3%) (Table 4). The system could detect more than 100% of the inhalation peak if the breathing cycle included local maximums.
Performance of SGD Versus RPM
In 5 of 39 patients (12.8%; 95% CI, 4.2%–30.0%), the following parameters were altered and considered SGD failures: quality only (dCycle, 1 patient), accuracy only (dtSGD, 2 patients), and both quality and accuracy (dCycle + dtSGD, 2 patients). As illustrated in Figure 3, the SGD allowed display of gated PET/CT images on software that is already commercially available. The SGD failed to generate a gated BTV (interoperability failure) in 1 of the 21 patients with increased 18F-FDG uptake.
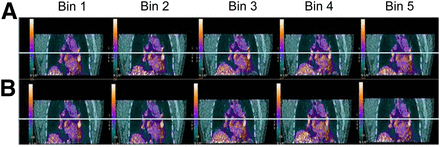
Simultaneous respiratory signal processing with SGD and RPM PET/CT. Patient was referred for suspected colorectal cancer recurrence with suggestive CT findings (1 left perihilar lung nodule). Shown are 2 reconstructed image data sets (SGD [A] and RPM [B] gated bins using AdvantageSim 4D software on Advantage Windows workstation). 18F-FDG PET/CT showed increased, nonspecific uptake bilaterally in (peri)hilar (L > R) and subcarinal lymph node regions on both SGD and RPM image datasets without significant differences in SUVmax, but without significant respiratory motion.
To summarize the SGD failures, 7 of the 40 patients (17.5%; 95% CI, 7.0%–36.1%) experienced failure: 1 patient regarding tolerance; 5 patients regarding quality and accuracy, using severe criteria; and 1 patient regarding interoperability.
Comparative data for the SGD against RPM are presented in Tables 4 and 5. The SGD significantly improved detection of peak inhalation (P < 0.05), by a median of 8.0%. The SGD also reduced the time lag between the detection of the peak and the trigger by a median of 260 ms (P < 0.05); baseline drift was also smaller with the SGD (P < 0.05). Had the same feasibility criteria been applied to RPM, all 39 cases would have been considered failure in terms of quality or accuracy (only accuracy, n = 26; both quality and accuracy, n = 13). RPM failed to provide gated BTV in 3 of 21 patients. Gated BTV and SUVmax did not significantly differ between the 2 gating devices (P = 0.18, the difference of gated BTV between SGD and RPM; P = 0.2, the difference of SUVmax between SGD and RPM). No 18F-FDG–negative lesions on ungated PET/CT became 18F-FDG–positive after gating, regardless of the gating technique
Performance of SGD Versus RPM
Figures 3 and 4 present 2 examples of interoperability with a commercially available platform.
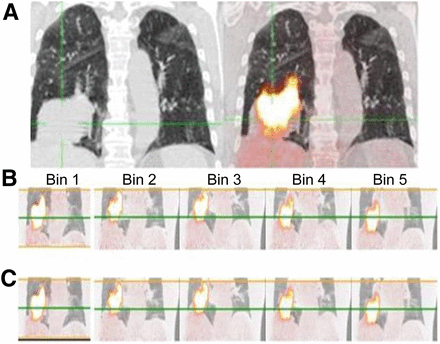
Simultaneous respiratory signal processing with SGD and RPM 18F-FDG PET/CT in 70-y-old man with T3N0 squamous cell carcinoma of inferior lobe of right lung who underwent imaging for staging. (A) Ungated PET/CT image (right) showing substantial misregistration due to respiratory artifacts and high tumoral uptake (body-weight–normalized SUVmax of lesion is 14.6). (B and C) SGD and RPM-gated PET/CT images, respectively, with body-weight–normalized SUVmax of 19.4 for SGD and 18.3 for RPM. Green line is reference level highlighting respiratory motion between bins. Supplemental Video 1 shows the displacement of the tumor associated with the patient’s breathing (top scanner, PET in the middle, and fused image at the bottom) on transverse views passing through the lung (left), in profile (at center), and coronal (right).
DISCUSSION
This prospective clinical study confirmed that in synchronizing PET/CT with respiration, the SGD was better tolerated and performed better than RPM and the SGD allowed for the generation of gated images on commercially available software.
The use of a facial mask was well tolerated by almost all patients (97.5%), who appeared to be a fair representation of the population in a real-life clinical setting. Improved reproducibility and correlation of respiratory volume over abdominal height with tumor displacement had already been demonstrated by x-ray fluoroscopy (14), with a shorter time lag (10), but these systems were developed for CT (10,16) or PET (12,25) and have not been used to synchronize PET and CT together. The reliability of respiratory gating data depends on the rate at which peak inhalation is detected (quality) and the time lag between this detection and the trigger (accuracy). Because breathing patterns in patients are often irregular, improvement of binning accuracy and reproducibility is a critical issue (25). In this population, the SGD outperformed RPM for these technical parameters. Nevertheless, these findings need to be validated in further clinical evaluation studies alongside evidence of clinical benefit.
The main drawback of spirometric measurements is the drift of the volume baseline, as was previously demonstrated in a 4-dimensional CT study (15). This drift with turbine-based pneumotachographs (9–11) is mainly due to breathing asymmetry (17). Therefore, pitot-tube–based pneumotachography (preVent; Medgraphics) is used for breath-hold radiotherapy (16), but this technique is also subject to drift originating from flow measurement errors. Signal drift for a very low flow (i.e., breath-hold) has been reported by the manufacturer but should not be a concern in normal breathing. In our study, we observed a small-volume baseline drift (median, 8.6%), but this drift was still twice lower than that obtained with RPM. Another type of possible signal drift is due to a shift in the sensor temperature, which we managed to control.
In this cohort of patients and with specific criteria, the SGD outperformed standard RPM. However, the clinical value of increasing the quality and accuracy of gating remains to be established. It is usually assumed that respiratory gating improves PET image quality and accuracy (26–28), but the additional patient radiation exposure from use of an extra gated acquisition should also be kept in mind.
Our BTVs and SUVmax did not significantly differ between gating devices. However, this study was not designed to address this issue; as a consequence, only 21 of 46 patients had pathologic 18F-FDG uptake, impairing the statistical power of this subanalysis. Moreover, 58% of lung nodules were in the upper part of the lung, which is less susceptible to inaccuracies caused by breathing movement (29). Therefore, further evaluation of the superiority of SGDs over RPM is warranted in terms of the accuracy of gating, the evaluation of SUV and BTV, and subsequent clinical benefits regarding lesion characterization and segmentation.
CONCLUSION
The feasibility of an SGD device was validated for PET and CT of the lung. The high patient tolerance, good technical quality, accuracy, and interoperability of this modality make it compatible with daily clinical use. The technical superiority of SGDs over RPM should be further investigated, as well as the translated clinical benefit of acquiring improved gated PET/CT data.
DISCLOSURE
This work was supported by a consortium of Institut Claudius Regaud, ISP system, GW Healthcare, and Toulouse III University, with funding from the Direction Générale de la Compétitivité de l’Industrie et des Services and the Midi Pyrénées Regional Council. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Anne-Laure Fize and Dr. Caroline Charles for their support of the work generated for this article.
Footnotes
Published online Apr. 24, 2019.
REFERENCES
- Received for publication November 13, 2018.
- Accepted for publication February 20, 2019.