Abstract
Nuclear medicine technologists are specialized health professionals who cover a wide range of tasks from clinical routine (including image acquisition and processing, radiopharmaceutical dispensing and administration, patient care, and radioprotection tasks) to leading clinical research in the field of nuclear medicine. As a fundamental concern in all radiation sciences applied to medicine, protection of individuals against the harmful effects of ionizing radiation must be constantly revised and applied by the professionals involved in medical exposures. The acknowledgment that nuclear medicine technologists play a prominent role in patient management and several procedural steps, both in diagnostic and in therapeutic nuclear medicine applications, carries the duty to be trained and knowledgeable on the topic of radiation protection and dose optimization. An overview on selected topics related to dose optimization is presented in this article, reflecting the similarities and particularities of dose reduction–related principles, initiatives, and practicalities from a global perspective.
The present article is the result of a consultation consortium involving the European Association of Nuclear Medicine Technologist Committee, the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Technologist Section, the Australian and New Zealand Society of Nuclear Medicine Technologists Special Interest Group, and the Canadian Association of Medical Radiation Technologists.
This global initiative on the topic of dose optimization is a pioneering and fundamental tool to understand how each of the leading associations of nuclear medicine is handling this topic.
The predicted outcomes from this project are to explore and describe the existing systems of dose optimization and to provide a comprehensive description of dose optimization methods, presented from a technologist point of view, aiming to inform and raise awareness among technologists. Controversies will be presented and explained, and consensus areas will be acknowledged.
According to a thorough literature research, the authors have identified several topics or population groups to which radiation optimization is critical, given either the acknowledgment of a higher susceptibility to radiation exposure, an observed increased frequency of a certain technique, or the recent introduction of a procedure into practice.
DOSE REDUCTION PRINCIPLES
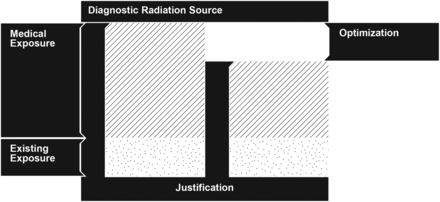
Nuclear medicine technologists and radiographers are responsible in most departments for preparing radiopharmaceuticals and performing imaging and, as members of the clinical team, are at the forefront of patient handling and care. All nuclear medicine procedures must be justified, as demanded by the principles of radiation protection of patients and workers (1). The justification principle is clearly stipulated in evidence-based peer-reviewed guidelines, allowing a topologic approach, on the clinical routine. The justification principle is aimed at eliminating the practice of unnecessary medical exposures. Optimization, however, might be seen as the necessary amount of radiation exposure to achieve a clinical outcome, given a set of technologic resources and patient attributes. This appropriation of the ALARA principle (as low as reasonably achievable) allows for a patient-specific approach but represents a considerable effort to determine the “right” technical conditions to attain the patient-tailored exposure optimization. To illustrate this concept, consider a nuclear medicine schematized diagnostic intervention in terms of radiation exposure (Fig. 1). As a baseline situation, the patient is exposed to that radiation of the general public (i.e., existing exposure). If the clinical benefit of this procedure is considered to surpass the risk of developing exposure-related diseases, then the justification condition is met. Given a set of methodologic and technical conditions involved in the intervention, it may be possible to reduce the medical exposure to attain the requested diagnostic outcome.
Depiction of justification and optimization principles in a situation of medical exposure.
Good practice leading to dose reduction is a complex multidisciplinary effort that includes accuracy in the clinical information needed for any nuclear medicine procedure. Determination of sufficient image quality with diagnostic potential, optimization of the quality of both components of hybrid imaging, and minimization of the radiation dose to the patient together with operator risk exposure are fundamental aspects of good clinical practice. Simultaneously, attention must be placed on patient comfort and respect for the department’s daily schedule, which ultimately is also aimed toward improved patient care.
Each step in every nuclear medicine procedure may be optimized with respect to external guidance (e.g., national law or association guidelines), starting with the radiopharmaceutical choice through the choice of different imaging protocols and always respecting the fundamental principles of the radiation protection system (2). Table 1 illustrates a generalized methodology to optimize dose. Depending on the available means provided to technologists and the critical thinking toward those means and possible alternatives, a suited optimization solution should be achieved.
Optimization Process Applied to Different Procedural Steps Accompanied by Operational Questions and Clarification Resources
The available means for dose optimization explored in this paper may be classified with respect to their origin:
Detection technology: crystal detector design and performance; exposure modulation CT
Computer technology: reconstruction algorithms
New technology: PET/MR and positron emission mammography; semiconductor detector technology for SPECT
Quantities related to lower doses: CT (mAs, axial field reduction); radiopharmaceuticals (activity)
Structural and behavioral issues: occupational exposure; department design
PEDIATRIC PATIENTS
From 2010 to 2012, the PEDDOSE.NET (Dosimetry and Health Effects of Diagnostic Applications of Radiopharmaceuticals with particular emphasis on the use in children and adolescents) project succeeded in identifying a series of challenges and necessary efforts to optimize exposures in nuclear medicine procedures (3). Emphasis was placed on dose reduction techniques, particularly on the fact that the instrumental technologic developments can be used to reduce patient radiation dose. With the development and progressive implementation of PET/MR scanners, a significant reduction of 18F-FDG activity can be achieved (4), and it is strongly encouraged that retrospective-dosimetry and image-quality studies be performed with emerging radiopharmaceuticals and radionuclides (5).
Optimizing the administration of radiopharmaceuticals to children requires a careful examination of the tracer’s radiation quality, biodistribution, and the child’s weight or body mass index. Therefore, recent pediatric tables were developed by the European Association of Nuclear Medicine (EANM) in an effort to harmonize a maximum of established radiopharmaceuticals in one system (6). Furthermore, the North American colleagues have developed a set of suggested radiopharmaceutical activities for pediatric patients (7).
In 2016, the SNMMI published a North American consensus guideline for pediatric administered radiopharmaceutical activities (8). The intent was to participate in the Image Gently Campaign and allow for high-quality images at low radiation dose based on weight per radiopharmaceutical.
Future iterations of both documents will include situations in which one or the other system provides advantages. The identification of differences from both guidelines should provide new opportunities for dose optimization and dose reduction in pediatric patients (8–10).
HYBRID IMAGING
PET/CT
PET/CT is the leading method for the diffusion of multimodality imaging in nuclear medicine (11). Combined PET/CT has increased diagnostic value, but it is commonly associated with a general increase in the radiation dose received by the patient (12). To be competitive with the evolution of nonionizing imaging techniques, PET/CT needs to develop constantly, with dose reduction as one of the main goals (13–15).
The above factors along with other factors such as legal aspects and a general increased fear and discomfort toward radioactivity in the population has pushed nuclear medicine toward a strong internal debate over dose reduction for patients and operators (16).
It is fundamental in every PET center, when creating acquisition protocols, to find a compromise between providing the best diagnostic-quality imaging while optimizing the dose to the patient.
In PET/CT daily practice, dose optimization goals are not only to expose patients to the lowest dose possible but also to produce a good technical-quality image. The coefficient for effective dose from 18F-FDG in adults is 1.9 × 10−2 mSv/MBq according to International Commission on Radiological Protection publication 128; that is, about a 3.5 mSv whole-body dose for an administered activity of 185 MBq (17).
EANM guidelines on PET/CT have the purpose of assisting in the practice of performing, interpreting, and reporting scans, and they can also be used for dose optimization by providing recommendations for 18F-FDG administered activity and CT dose (18).
Although it is generally accepted that CT dose reduction is accomplished through x-ray beam current modulation across the length of the patient, corresponding to measured patient width (19), additional discussion arises from injected activity. In recent years, advances in PET technology introduced the potential to lower injected activities while minimizing the impact on image quality. This was achieved primarily through improved hardware capabilities and design, such as increased scanner sensitivity from additional detector rings and time-of-flight capability. As an example, in version 2 of the EANM procedure guideline for 18F-FDG PET imaging of tumors (14), a minimum recommended administered 18F-FDG activity is defined, but a higher activity may be administered to reduce the duration of the PET scan. To a certain extent, it is preferable to use a reduced activity and increase the study duration, thereby applying the ALARA principle, and keeping in mind the effect on patient comfort (longer scans) and a department’s workflow.
In the guideline, recommendations are provided for determining the minimum 18F-FDG administered dose in adults—recommendations that assume a linear relationship between PET acquisition time per bed position, patient weight, and recommended 18F-FDG activity.
With a linear relationship for systems that apply a PET bed overlap of no more than 30%, the minimum recommended administered activity is calculated as follows:
For systems that apply a PET bed overlap of more than 30%, the minimum 18F-FDG administered activity is calculated as follows:
An alternative quadratic relationship is also provided in the document and results in a slightly higher administered activity for patients weighing more than 75 kg.
For patients weighing more than 90 kg, increasing the emission acquisition time per bed position rather than increasing the administered 18F-FDG activity is recommended to improve image quality. The literature suggests that 18F-FDG activities higher than 530 MBq for patients above 90 kg should not be applied for lutetium yttrium orthosilicate and lutetium orthosilicate systems (14).
It is possible that a maximum administered 18F-FDG activity may be imposed by national law. If the PET acquisition duration for each bed position can be set separately, this then may be further reduced by up to 50% outside the thorax and abdomen (i.e., at the level of the head, neck, and legs) because overall attenuation in these body regions is lower. The 18F-FDG activity must still be calculated using the longest acquisition duration for bed positions at the level of the thorax and abdomen. Systems with continuous motion functionality may increase motion speed 2-fold outside the thoracic and abdominal regions, rather than adjust the minutes per bed position.
An exploratory further optimization is presently being evaluated by EANM Research Ltd. This optimization would allow lowering the administered 18F-FDG activity for PET/CT systems with higher sensitivity or improved performance using new, enhanced technology (e.g., better time-of-flight performance, solid-state digital PET detectors, continuous bed motion, or extended axial field of view, i.e., length of bed position). A prerequisite is that imaging sites first obtain EANM Research Ltd. accreditation for that system (20,21).
SPECT/CT
In conventional nuclear medicine also, hybrid imaging has appeared as a valuable imaging tool. A simultaneous transmission scan often provides diagnostic differentiability and increased lesion detectability, accompanied by an increased radiation burden (22,23). Transmissionless attenuation correction techniques, such as the Chang method, may provide enough image compensation to achieve a satisfactory diagnostic image, most evidently in brain imaging (24). Other dose optimization techniques include a selection of the lesion anatomic region by means of the SPECT sinogram, which is used to delimitate the low-dose CT (25). This technique is useful for differentiation between bone degenerative focal lesions and bone metastasis, with a reduced tube current and scan length.
CARDIAC IMAGING
Dose optimization in myocardial perfusion imaging (MPI) is a complex topic with several aspects that need to be considered. The general strategy for dose reduction proposed in this paper can be applied, integrating the specificities of MPI (26–29).
According to the European Council Directive 2013/59 (1), the optimization must take into account the current state of technical knowledge, including selection of equipment, to obtain a clinical diagnosis. A great number of factors are considered in the selection of the equipment in nuclear cardiology (30).
The initial step for MPI is selecting the radiopharmaceutical on the basis of the justification principle. This is where the optimization process should start: 99mTc-based tracers guarantee a reduced patient radiation exposure compared with 201Tl. Cardiac PET tracers can further reduce the exposure compared with SPECT tracers (31). This means medical exposure can reasonably be reduced with the right radiopharmaceutical selection, in accordance with the ALARA principle, when societal and technologic conditions allow.
Protocol selection is another key strategy in dose optimization. Ideally, the stress study should be performed first, since the rest study can be omitted if the stress study shows normal perfusion, left ventricular function, and wall motion on physician review before rest imaging (32). This imaging strategy significantly reduces radiation exposure to the patients (33). Eliminating the rest study also reduces the dose to the practitioners (34). The dose reduction to staff and patients can be further improved by switching from a fixed-activity protocol to a weight-based adjusted radiotracer amount while preserving image quality (35).
In the nuclear cardiac imaging context, the injected activity also depends on the imaging instrumentation: scintillation camera or a cadmium-zinc-telluride detector, imaging time, pixel size, gated acquisition, reconstruction algorithms. Advances in technology have greatly contributed to nuclear cardiology and highly influenced the dose reduction. Nevertheless, it is not possible to precisely quantify and standardize the injected activities; they must fit the software- or hardware-based features of the available instrumentation in the laboratory. The practitioner must be aware of the instrument’s operating performance and how to adapt it to the best practice procedures.
Dose optimization software tools for SPECT are based mainly on iterative reconstruction algorithms with resolution recovery. It is suggested that this reconstruction modality can allow a reduction of half the injected dose compared with that of filtered backprojection or conventional iterative techniques (36,37). The iterative reconstruction algorithms with resolution recovery can be combined with hardware components such as a dedicated multifocal collimator, a cardiocentric acquisition, which allows the use of either a low-dose or a short-time imaging protocol, or a combination of the two (38,39).
Technologic advances in hardware have led to the development of solid-state detectors. The currently available cameras use squared cadmium-zinc-telluride crystals, which allow a greater count sensitivity (40). This technology outperforms the sodium iodide crystal detector scintillation camera, allowing a further dose reduction to weight-adjusted activity (41,42), leading the practitioner to reconsider whether use of sodium iodide crystal detectors is still the best clinical practice.
Among the hardware solutions, despite controversial issues (43), attenuation correction for MPI deserves a mention. The attenuation correction is particularly valuable in the setting of stress-only MPI, reducing the need for additional rest imaging by roughly a third (44) at the cost of a low-dose x-ray CT scan (45,46). CT attenuation correction plays a role in dose optimization for those patients in whom the stress scan has sufficient clinical information to avoid rest imaging, which would carry a 3-fold activity increase compared with stress imaging.
PET MPI also benefits from the improved technology. The availability of a 3-dimensional acquisition protocol is preferred to a 2-dimensional one. The sensitivity of 3-dimensional systems, without interplane septa, offers significantly higher-count imaging and the radiation dose can be further lowered (47); this implementation allows myocardial blood flow measurements (48). PET MPI only recently became practical because of the improved timing resolution achievable by new coincidence electronics combined with fast scintillators (lutetium orthosilicate, lutetium yttrium orthosilicate) and the development of time-of-flight and point-spread-function modeling. The combination of the time-of-flight reconstruction and point-spread-function modeling shows improved image quality (49,50). The point-spread-function and time-of-flight combination is useful for the qualitative assessment and implementation of a low-activity protocol. Nevertheless, the different reconstruction methods may have a severe impact on quantitative assessment of the myocardial blood flow and on its standardization (51). Because of this issue, the reconstruction protocol for quantitative evaluation, including the point-spread-function and time-of-flight use in myocardial blood flow reconstructed images, should follow the manufacturer recommendations (52). Concerning cardiac PET imaging, recent papers (53,54) have shown the high performance of PET/MR in this field. Making use of the justification principle, one could consider PET/MR as a means to avoid the CT exposure from PET/CT.
A final strategy always effective to reduce the dose is to increase the patient’s hydration and early micturition after radiopharmaceutical administration (55).
NEW TRENDS IN RADIONUCLIDE THERAPY
Dose optimization is an important tool to treat patients with an effective dose to the target volume and an ALARA dose to the nontarget volumes.
99mTc-macroaggregated albumin used for pretreatment 90Y radioembolization therapy is recommended for a personalized approach in patient selection and personal oncologic distribution as well as a pretherapeutic predictor of response (56).
Dose optimization during peptide receptor radionuclide therapy is achieved by performing a good investigation before and during the treatment. Patients have to be selected by PET scans to predict the tumor load, and the kidney function has to be assessed, measuring the glomerular filtration rate, to avoid severe nephrotoxicity. Depending on the radionuclide used, 177Lu or 90Y, dosimetry has to be performed before or during the therapy. Based on the results of the dosimetry, the given activity can be personalized in order not to damage the kidneys or undertreat the patients (57).
After treatment, a long-term follow-up of the kidney function, using 99mTc-diethylenetriaminepentaacetic acid or 51Cr-ethylenediaminetetraacetic acid, is advised to monitor for nephrotoxicity (58).
Most PET tracers do not have a long enough half-life to be used to determine tumor and normal-tissue dosimetry to get the ideal therapeutic dose. It is common practice to administer a standard dose of 177Lu (7.4 GBq) and use either multiple SPECT scans or one SPECT scan in combination with whole-body 177Lu data to determine tumor uptake, washout characteristics, and absorbed doses. Doses for subsequent treatments can be adjusted on the basis of tumor burden and dose to normal tissue.
Given that individualized dosimetry is recommended and will be included in the new European Council Directive 2013/59 (1), it is noted that in daily practice absorbed dose planning is rarely performed. The multiple imaging, blood sampling, and subsequent results processing are time-consuming and present the main obstacle for routine implementation (59,60).
PET OCCUPATIONAL EXPOSURE
Unlike conventional nuclear medicine, technologists working with positron-emitting radioisotopes cannot decrease their body exposure to these high-energy PET radiotracers using lead aprons (61). Because positron-emitting radioisotopes have greater than 10 times the half-value layer of 99mTc (62), there is no functional body shielding that can be worn. For a PET technologist, in addition to dose optimization, minimizing occupational exposure depends on time, distance, and shielding of the dose-drawing and dose-injecting apparatus.
Multiple published articles have found that the task resulting in the highest radiation exposure in a PET clinic is dose administration (63–65). With this result in mind, departments should focus on improving shielding in the dose-drawing area and when injecting patients. Departments may choose to design their own dose-drawing stations using options such as lead bricks, PET L-blocks, or other PET isotope dose-drawing devices, or departments may acquire automated dose injectors for dose drawing and injecting. A department that uses an autoinjector or automatic dispenser reported a 10-fold decrease in staff extremity and body doses when administering 18F-FDG (66).
Both increasing the distance from patients during the injection and reducing the time spent while injecting contribute to lowering the radiation exposure during dose administration. Since the time spent handling the radioactive syringe has such an impact on overall exposure, wearing a ring dosimeter on both hands could help a department analyze its procedures and the individuals’ techniques and improve the overall design (65,67). A change in shielding plus reviewing each technologist’s dose-drawing technique, to find the quickest and most efficient dose-drawing method, might help lower an individual’s exposure in one or both hands plus lower the department’s overall extremity exposure.
Handling high energy β-emitters, for imaging (e.g., 68Ga) or therapy (e.g., 90Y), does present the potential for increased extremity exposure (68). The introduction of simple cold kit labeling with 68Ga (69), which can be performed by the technologist, requires dose optimization and must be performed with appropriate training and shielding equipment. Additionally, as the dose limitation for eye lens exposure has recently been reviewed (1), eye lens monitoring is recommended for those technologists who handle high-energy β-emitters on a regular basis. Additional eye protection (e.g., x-ray goggles) can be considered to keep the radiation exposure at an acceptable level (70).
When in proximity to radioactive patients during the scanning phase, distance and time become the crucial ALARA principles to follow. Time must be minimized and distance increased without impacting patient care; a patient’s safety and feeling of safety should not be compromised, nor should the patient feel alienated because of being radioactive. It is essential to carefully consider patients’ well-being while minimizing technologists’ radiation exposure in each unique situation.
EANM INITIATIVES FOR DOSE OPTIMIZATION
Being the scientific reference of nuclear medicine in Europe, the EANM has been involved in the most relevant European Union–sponsored initiatives and projects. Relevant to the medical technologic practice of nuclear medicine is EANM participation in the European Alliance for Medical Radiation Protection Research, with the goal of improving medical care and radiation protection through sustainable research (71,72).
Additionally, the Multidisciplinary European Low Dose Initiative, which EANM joined in 2016 in a coordinated effort to research the effects and health risks after exposures to low-dose radiation (73,74), provides valuable research resources for nuclear medicine technologists. Another EANM consortium specifically for the investigation of low-dose medical exposures, the MEDIRAD project, aims to increase the scientific bases and clinical practice of radiation protection in the medical field both in diagnostic and in therapeutic applications.
Recognizing the momentum and increasing concern regarding radiation protection and optimization, the EANM created a new committee dedicated to radiation protection in 2016.
The EANM Technologist Committee has also acknowledged the importance of dose optimization, publishing one edition of the annual technologist’s guide on the topic of radiation protection and dose optimization (75).
AUSTRALIAN AND NEW ZEALAND SOCIETY OF NUCLEAR MEDICINE INITIATIVES FOR DOSE OPTIMIZATION
The Australian Radiation Protection and Nuclear Safety Agency recently undertook a survey of Australian nuclear medicine, PET, and radionuclide therapy patient doses and released an updated diagnostic-reference-level guideline based on the 25th, 50th, and 75th percentile administered activity (as used by the International Atomic Energy Agency) (76–78). Sites were able to generate a report that compared the sites’ standard administered activities with the Australian mean and median doses, allowing sites to revise and optimize doses based on the current best practice. The guidelines also include volume CT dose index for a CT study performed on a hybrid PET/CT or SPECT/CT device for the purposes of attenuation correction and anatomic localization (low-dose CT). The new diagnostic reference levels have been endorsed by the Australian and New Zealand Society of Nuclear Medicine, the Australasian Association of Nuclear Medicine Specialists, the Royal Australian and New Zealand College of Radiologists, and the registration and accreditation boards (77,79). Pediatric doses are currently under review, with the guidelines to be released late 2018.
Occupational exposure is overseen both by the Australian Radiation Protection and Nuclear Safety Agency and by state-based radiation control boards. The code of practice for exposure to ionizing radiation was revised in 2016 to reduce the occupational eye lens exposure to 20 mSv annually, with a 3-y exposure of less than 50 mSv (as per International Commission on Radiological Protection recommendations) (77,78,80,81). The use of lead goggles for technologists, radiographers, radiologists, and radiopharmacists (especially during the manufacture and dispensing of therapeutic tracers such as 177Lu, 67Cu, and 90Y) is recommended. The Royal Australian and New Zealand College of Radiologists has released recommendations on appropriateness criteria for referrers and those working in the profession to reduce the number of potentially unnecessary tests involving ionizing radiation being performed. The aim is to reduce radiation exposure to patients and staff, as well as the financial burden on the health service of doing expensive procedures without justification. The guidelines are being reviewed to include better criteria for nuclear medicine and PET procedures. Online tools for appropriateness criteria are currently being tested in multiple large teaching hospitals in Australia, with a plan to make them available to referrers. The tools would also include information on radiation exposure to the patient, including the ability to calculate lifetime cumulative patient dose.
SNMMI INITIATIVES FOR DOSE OPTIMIZATION
The SNMMI, based in the United States, has written a position paper on dose optimization (82) and devoted an entire section on its website to dose optimization (83). On this site, there are many useful tools, including links to recent articles on dose optimization from both The Journal of Nuclear Medicine and the Journal of Nuclear Medicine Technology. There are links to both the Image Gently (84) and the Image Wisely (85) campaigns, which have focused on dose optimization and its importance to pediatric dose and adult dose and appropriate imaging scans. The right scan for the right patient with the right amount of dose for optimal imaging quality is the mantra going forward. Another useful freely available resource is the Nuclear Medicine Radiation Dose Tool (86). With this tool, one can input the type of study and the patient model (based on age and category) and get a recommended minimum and maximum dose range for that study along with a dose estimate. This tool can be useful in dose optimization and is available for free online. In addition, the SNMMI Technologist Section has developed 2 books concerning myocardial imaging and abdominal imaging on quality, safety, and dose optimization (87,88).
CONCLUSION
In an effort to describe the current global technologist involvement in dose optimization, this document provides harmonized definitions for concepts fundamental to the practice of dose optimization in the context of nuclear medicine. The current description of the available underlying literature provides fundamental support for evidence-based application of the agreed-on principles.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Footnotes
Published online Nov. 9, 2018.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.
- 28.
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- Received for publication July 20, 2018.
- Accepted for publication September 25, 2018.