Abstract
Clinical protocols play an important role in the provision of high-quality care in nuclear medicine. Properly written protocols help to ensure that nuclear medicine procedures are performed in a standardized, reproducible manner so that patients receive high-quality care. The following article is the second in a 2-part series on how to write a protocol. A framework for composing protocols and the components of clinical imaging protocols were detailed in the first article. This article details the framework and components of protocols for cardiac stress testing, therapy, and quality control.
The following article is the second in a 2-part series on how to write a protocol. As discussed in part 1, protocols play an important role in the provision of high-quality care in nuclear medicine. Properly written protocols help to ensure that nuclear medicine procedures are performed in a standardized, reproducible manner. Site-specific, detailed protocols are important not only for diagnostic imaging protocols but for other protocols as well. Other types of protocols should follow the same general principles, as well as being based on the evidence from professional society guidelines and peer-reviewed published literature. This article will detail the protocols for cardiac stressing testing, therapy, and quality control.
The key components of a protocol vary depending on the type of protocol. For instance, the elements necessary for a clinical imaging procedure are different from the elements necessary for a stress testing procedure. There may be elements of a therapy protocol that are not necessary for a diagnostic imaging examination. Likewise, the components of a quality control protocol will be different, with specific details to allow precise reproduction of the test. The necessary components for each type of protocol are described below.
CARDIAC STRESS PROTOCOLS
There are specific components that must be included for both exercise and pharmacologic stress protocols (1). Along with a general requirement for being detailed and systematic, cardiac stress protocols also must include instructions for coordination with supporting departments when stress procedures are performed outside the nuclear medicine department. Comprehensive stress protocols are necessary to ensure the proper timing of administration of the radiopharmaceutical relative to the performance of stress testing. An error frequently encountered when exercise stress testing is performed without direct oversight from the nuclear medicine department is tracer injection as soon as the patient achieves 85% of the maximum predicted heart rate instead of waiting for the test to be symptom-limited and maximum coronary artery dilation to be present (2).
Components of exercise and pharmacologic stress protocols include the following (Table 1).
Required Protocol Elements
Graded/Infusion Protocol
The protocol should include a detailed description of the exercise protocols and when they are used. For instance, relatively healthy patients in fair shape may be exercised using a Bruce protocol. However, elderly or deconditioned patients should be exercised according to a modified Bruce or Naughton protocol (3). The protocols should contain charts detailing the speed, incline, and workload for each exercise protocol stage.
For pharmacologic stress testing, the infusion technique should describe the stress agent (i.e., adenosine, dipyridamole, regadenoson, or dobutamine), dose with calculation instructions as appropriate, and infusion length. For adenosine and dipyridamole stress protocols, dosing charts based on weight are helpful to include in the protocol.
If low-level exercise is performed in conjunction with pharmacologic stress, the protocol should describe the speed and elevation of the treadmill and duration of the low-level exercise.
Timing of Assessments
As part of the step-by-step details, the stress protocol should indicate the time for the measurement of heart rate, blood pressure, and electrocardiography tracings. Commonly, the heart rate is assessed and electrocardiography tracings documented at the completion of each minute of exercise stress whereas the blood pressure is assessed at the end of the second minute of each stage. The instructions should also include the timing for assessment of symptoms such as fatigue or shortness of breath.
There should also be instructions for poststress monitoring, again for the timing of assessment of heart rate, blood pressure, electrocardiography tracings, and symptoms. Criteria for terminating poststress monitoring should also be stated, such as the patient’s return to baseline heart rate and blood pressure.
Injection Criteria
The stress protocol should clearly indicate the criteria for injecting the tracer along with exercise and testing endpoints, including any specific events that are reasons for stopping the stressing activity. For pharmacologic stress, the timing of tracer injection must be specified. The differences in instructions between a dipyridamole and adenosine stress protocol demonstrate this point clearly. For dipyridamole stress, dipyridamole is infused over 5 min and the tracer is injected 2 min later at peak hyperemic effect. In contrast, when adenosine stress is performed, the adenosine is infused for 2–3 min and the tracer is injected at peak hyperemia but the infusion is then continued for another 2–3 min because adenosine has such a short time of effect.
Reasons for Early Termination of Stress
Because there are potential risks associated with exercise and pharmacologic stress, warning signs or reasons to consider early termination of the procedure should be listed in the protocol and clearly understood. For instance, a clear reason for early termination and reversal of dobutamine stress is a decrease in the patient’s systolic blood pressure of greater than 20 mm Hg from baseline when accompanied by other evidence of ischemia.
Identification and Treatment of Adverse Reactions
Finally, the protocol should clearly list the common side effects associated with the type of stress along with appropriate treatment so technologists recognize them and are prepared to act promptly. Technologists are readily aware of the side effects associated with dipyridamole along with treatment with 125–250 mg of aminophylline intravenously. However, the common side effects of exercise and their treatment are often not included in the protocol but are equally important. These include the administration of 0.4 mg of sublingual nitroglycerin for chest pain or 2–6 L/min of oxygen for shortness of breath.
THERAPY PROTOCOLS
Many of the same elements for clinical imaging protocols are also required for therapy protocols. These include clinical indications and contraindications; patient preparation, education, and instructions such as diet restrictions and the withholding or nonwithholding of medications; radiopharmaceutical identity, dose range or method of calculation, and route of administration; and nonradioactive or adjunct medications. Additional elements that must be described include the requirement for a written directive, a treatment procedure, and any imaging protocol after therapy (if necessary). The requirements for therapy protocols are detailed in Table 1.
Indications and Contraindications
Each therapy protocol should have specific indications and contraindications stated in a simplified manner. Clinical indications list the reasons why the therapy may be necessary based on clinical evidence.
Contraindications are the specific reasons or situations in which a therapy procedure should not be performed because it may be harmful to the patient or there may be no benefit. For instance, administration of 89Sr-chloride (Metastron; GE Healthcare) for palliation of bone pain in patients with a life expectancy less than 4–6 wk is contraindicated because of the 1- to 3-wk time to effectiveness of the treatment and the radiation safety precautions required should death occur in the first week of therapy (4). It is critical for technologists to pay attention to contraindications when performing therapy procedures because of the serious biologic effects of therapeutic radionuclides.
Patient Preparation, Education, and Instructions
Appropriate patient preparation before therapy procedures is crucial to the effectiveness of the therapy. All protocols should specifically list requirements for food or diet restrictions. The protocol should indicate whether the patient may eat or drink and for how long. Protocols should specifically state whether certain foods must be avoided before the test and the specific period for abstinence. For example, 131I-NaI therapy for thyroid disease is more effective for patients who have followed a low-iodine diet 7–14 d before dose administration.
The withholding or nonwithholding of medications can be vital to the effectiveness of a therapy. Again, effective outcomes for 131I-NaI therapy require the discontinuation of iodide-containing medications (over-the-counter and prescribed medications) and thyroid supplements for a prescribed time based on the treatment protocol.
Protocols should also provide patient instructions for specific medications administered before the test. For example, therapy for B-cell non-Hodgkin lymphoma with 131I-tositumomab (Bexxar; GlaxoSmithKline) or 90Y-ibritumomab tiuxetan requires the patient to be premedicated with acetaminophen and diphenhydramine 30–60 min before administration of the cold antibody (tositumomab or rituximab).
It is also helpful to detail other patient instructions in the protocol, such as for multiday procedures. Once again, depending on the selected therapy protocol for B-cell non-Hodgkin lymphoma, diagnostic imaging followed by administration of the therapeutic radiopharmaceutical may be performed over a 7- to 9-d period.
Radiopharmaceutical Identity, Dosage, and Route of Administration
The administration of a radionuclide to a patient must be prescribed by an authorized user and include the identity, dose, and route of administration (e.g., oral, intravenous, or intraarterial).
Adjunct Medications
All adjunct medications used as part of the imaging procedure must be stated in the protocol. Again, the protocol should include the medication identity, dose, and route of administration along with the timing of administration, patient instructions, requirements for patient monitoring, and precautions for side effects or restrictions. An example of an adjunct medication used with a 131I-NaI therapeutic protocol is recombinant human thyrotropin (Thyrogen; Genzyme) as an alternative to thyroid hormone withdrawal.
Requirement for Written Directive
A written directive is a written order for the administration of a radiopharmaceutical to a specific patient and is required for the administration of 131I-NaI in amounts greater than 1.11 MBq (30 μCi) and therapeutic doses of any other radiopharmaceutical. The protocol should state the requirements for the written directive, including patient name, radiopharmaceutical identity, dose for the specific patient, route of administration, signature of the authorized user, and date. There should also be instructions in the protocol for either immediate verification or a time-out before dosing to verify that the administration is in accordance with the written directive (5).
Treatment Procedure
Pretreatment requirements must be detailed in the protocol to help ensure that they always occur. Instructions for the review of relevant clinical history, laboratory and pathology results, and imaging data should be included. There must be instructions for obtaining informed consent with an explanation of the risks, benefits, alternatives, and likelihood of success of the procedure along with documentation of the patient’s signature and date. There must also be instructions for verification that the patient is not pregnant or breast-feeding.
Importantly, the therapy protocol must also include specific detailed instructions for how and when the treatment is administered. Additionally, in many institutions, therapeutic protocols are performed using a multidisciplinary team approach including one or more of the following departments: radiation oncology, interventional radiology, hematology/oncology, radiation safety, and intravenous infusion. When a multidisciplinary approach is used, the therapeutic protocol should detail the responsibilities of each department or individual, coordination and timing of all activities, required documentation, and those responsible for patient monitoring and follow-up.
In addition to pretreatment and treatment instructions, therapy protocols must also state patient radiation precautions after treatment that are specific for the isotope administered. Common instructions stated in the protocol include the need to maintain distance (e.g., 1.83 m [6 ft]) from others, especially children and pregnant women, and to minimize time in public places; use of separate bathrooms and bedrooms; methods of safe travel, such as when using public transportation or crossing borders; control of bodily fluids and the handling of potentially radioactive household trash; frequency of hand washing; use of separate eating utensils; and separate washing of clothes. Finally, the duration of restrictions should be clearly spelled out.
If the treatment procedure requires the patient to be hospitalized, additional information must be included in the protocol. The protocol must include instructions for radiation safety for direct nursing and housekeeping staff, requirements for room signage, directions for radiation monitoring, rules for visitation, handling of materials used by the patient, release criteria, and response to medical emergencies or patient death (6).
Imaging After Therapy
If any imaging is required in conjunction with a therapy procedure (e.g., 131I-NaI posttherapy whole-body imaging), detailed imaging procedures must be described and include components as listed above in the section on clinical imaging protocols.
QUALITY CONTROL PROTOCOLS
A facility’s need to have detailed quality control protocols for all equipment is often overlooked. There should be specific written procedures for the performance of quality control for imaging equipment (γ cameras, PET scanners) and nonimaging equipment (probes, well counters, survey meters, xenon traps).
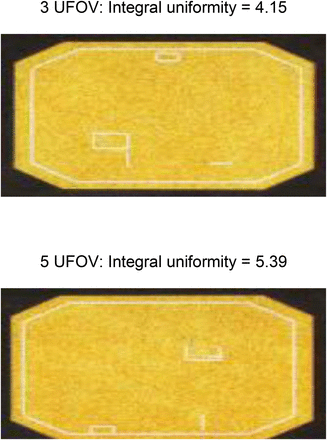
Detailed written protocols help to ensure that quality control tests are performed the same way by all technologists and that the results are comparable. For example, the procedure for performing an intrinsic flood test should specifically state the distance of the point source from the camera head (7). Differences in source-to-detector distance cause significant variation in the integral and differential uniformity results (Fig. 1). Without clear instructions, if 2 technologists use different source-to-detector distances, the resulting variations in integral or differential uniformity might be assumed to be the result of photomultiplier tube drift instead of simple procedural differences.
Demonstration of importance of standardized quality control protocols. Results of intrinsic integral uniformity values when point source is placed 3 uniform fields of view (UFOV) from face of camera and 5 UFOV from face of camera.
Site-specific and camera-specific quality control protocols should include the following (Table 1).
Frequency and Responsibility for Quality Control Tests
Protocol instructions must specify the frequency of quality control testing. Some quality control tests must be done daily, whereas others are done weekly, monthly, quarterly, or semiannually (8). The frequency of testing should follow the manufacturer’s recommendations along with the National Equipment Manufacturers Association requirements. The protocol should also identify who is responsible for performing the tests. Depending on the complexity of the test, this person may be the technologist, health physicist, or service engineer.
Type of Source and Position
Several types of sources are used to perform quality control tests. For γ-camera field uniformity, a facility may choose to use a 57Co flood source, a fillable 99mTc flood source, or a point source. Depending on the type of source used, the instructions should be specific. For instance, if the facility uses a fillable 99mTc flood source, there must be instructions for how much radioactivity to use and how to fill the flood source, including instructions for avoidance of air bubbles or overfilling and on uniform mixing.
The position of the source is also important and must be clearly defined. A source may be laid directly on the camera head or positioned at least 5 fields of view away. Other positioning instructions such as suspension of the source directly over the middle of the field of view to minimize scatter should also be included. For some cameras, the source must be placed in a holder provided by the manufacturer.
Equipment Setup
Similar to the camera setup for imaging protocols, the camera or equipment setup for quality control protocols must be clearly defined. These instructions should include such details as collimator use (intrinsic vs. extrinsic), collimator type, energy setting and window, matrix size, and zoom (9). It is not acceptable for a protocol to simply state “following manufacturer’s recommendation,” and most accreditation organizations will not accept a copy of the manufacturer’s written protocol in lieu of a site-specific protocol.
Acquisition Instructions
Again similar to the acquisition instructions for imaging protocols, the protocol must state acquisition instructions such as views or time/count per view. Also, if a phantom is used as part of the test, the protocol must state the type of phantom and instructions for use. For instance, the instructions for a resolution/linearity test should state whether a 4-quadrant bar phantom, parallel-line equal-spacing phantom, or orthogonal-hole phantom is used. There should also be instructions for whether the phantom should be rotated and how many times.
Processing Instructions
With modern cameras and equipment, the processing or evaluation of system performance is often automated. However, if applicable, there should be instructions for placement of regions of interest for quantification, sinogram generation, and creation of graphs. Specific instructions for correction values may also be needed.
Postacquisition Instructions
It is not enough to simply perform a quality control test and file the results away. Quality control results must be evaluated for abnormal equipment functioning and gradual deterioration of image quality. Protocols should define limits of acceptability for the results (tolerance limits) along with courses of action to be taken if the limits are exceeded. Corrective action may be as simple as rebooting the system or may require a visit by a field service engineer. Protocols should also contain instructions for the retention of quality control test results and comparison with previous results. For instance, when the results of a bar phantom test are evaluated, the smallest set of bars that can be resolved should be recorded. The images from the current test should be compared with previous images over time to determine whether there has been any deterioration in resolution.
CONCLUSION
Writing protocols that are detailed, unambiguous, and consistent is easier said than done. Protocols must be evidence-based, with meticulous attention to detail. Properly written protocols help to ensure that nuclear medicine procedures are performed in a standardized, reproducible manner so that patients receive high-quality care. Parts 1 and 2 of this article were designed to provide technologists with a framework for composing appropriate protocols for diagnostic imaging, cardiac stress testing, therapy, and quality control.
Footnotes
Published online Feb. 5, 2015.
CE credit: For CE credit, you can access the test for this article, as well as additional JNMT CE tests, online at https://www.snmmilearningcenter.org. Complete the test online no later than March 2018. Your online test will be scored immediately. You may make 3 attempts to pass the test and must answer 80% of the questions correctly to receive 1.0 CEH (Continuing Education Hour) credit. SNMMI members will have their CEH credit added to their VOICE transcript automatically; nonmembers will be able to print out a CE certificate upon successfully completing the test. The online test is free to SNMMI members; nonmembers must pay $15.00 by credit card when logging onto the website to take the test.
- Received for publication November 18, 2014.
- Accepted for publication November 19, 2014.