Abstract
The aim of this study was to evaluate the volume-of-interest (VOI) technique in the measurement of volume radioactivity and in the differentiation of necrotic sites from residual tumor activity in a phantom. Methods: PET/CT was performed on a phantom filled with 18F-FDG solution at different concentrations. The VOI was quantified in 2 sessions to evaluate the VOI measurements as a function of activity concentration in the phantom. Software was used to build the VOI, determine the volume radioactivity of the cylindric inserts (cm3), and compare them with their real volumes. The VOI technique was also used to discern the mixed distribution of regions of 18F-FDG activity from cold regions that represent areas of necrosis without tumor activity. Results: Volumes measured with the VOI technique were similar to the actual volumes of cylinders in the phantom (no statistical differences; P > 0.05 after t test analysis). The diameter of cold inserts correlated positively with the percentage of visualization (P < 0.01); in both sessions, it was possible to visualize 100% of the 12.7-, 11.1-, and 9.5-mm cold rods. Conclusion: VOI technique has shown great potential for evaluating volume radioactivity and differentiating hot and cold regions in a phantom; clinical studies should be performed with this technique to evaluate its utility.
The success of antineoplastic therapy requires accurate assessment methods that allow the clinician to make the best therapeutic decision. Initially, a response to treatment was detected by a reduction in tumor size (1). The World Health Organization originally published (2) the Response Evaluation Criteria in Solid Tumors (3). Furthermore, with the arrival of hybrid diagnostic methods such as PET/CT, it became evident that tumors may show a response to treatment without a decrease in size (4).
The response of tumor cells to chemotherapy or radiotherapy can be evaluated with PET/CT using 18F-FDG, which quantifies the actual extent of the tumor even when the tumor does not show a change in size, such as happens when tumor cells are replaced by fibrotic or scar tissue (5). PET/CT with 18F-FDG has become a tool to evaluate treatment response in malignancies such as Hodgkin lymphoma, in which a residual tumor mass remains after completion of the first line of treatment (6).
Currently, PET/CT is the most common method used to assess response to chemotherapy; however, PET/CT does not consider the tumor size (7). The maximum standardized uptake value (SUVmax) is a semiquantitative tool for PET/CT that permits the determination of 18F-FDG concentration in a specific region of interest. SUVmax correlates the injected dose of 18F-FDG, the patient's weight, and the lapsed time between injection and scanning (8). SUVmax is used to determine the therapeutic response by comparing metabolic activity at baseline with that after chemotherapy. Tumors that respond to treatment show a reduction in SUVmax that may be related to variables such as average time to progression and overall survival (9,10).
Some disadvantages of using SUVmax measurements are that they evaluate only a small area of the tumor (the region of interest), which may not represent the biologic behavior of the entire lesion (11). In addition, the evaluation matches with only 1 tumor, which is a limitation when the patient has multiple lesions and some decrease in tumor SUVmax may be observed. Also, controversial evidence has shown the inconsistency between the 18F-FDG concentrations in a tumoral mass, evaluated by SUVmax, and the survival rate in patients with cancer (12–14). In some cases, it has been suggested that treatment of patients should not be based on metabolic activity as assessed by the incorporation of 18F-FDG into the tumor (12,13,15).
The emerging use of volume of interest (VOI) has been of great interest for evaluating treatment response with 18F-FDG PET/CT (16–19). To measure tumor volumes in dedicated PET systems, some investigators have proposed the application of thresholding techniques that can be used later to estimate glycolysis activity in tumors (17–19). This glycolytic activity has been proposed as an index to measure and compare treatment responses of tumors; with modern hybrid PET/CT systems, VOI can be calculated in a direct way using the software tools of each commercial system.
VOI is a tool that determines the volume concentration of the radiopharmaceutical (volume radioactivity) in the entire tumor, including areas of different SUVmax, and represents them as a value in cubic centimeters. If the patient has more than 1 tumor, the VOI of each can be obtained, to focus on 18F-FDG, and then all VOIs can be merged to get the total tumor volume. This method can use PET/CT to estimate the amount of tumor in a patient at baseline and after treatment, providing simplicity in determining the response of a single tumor regardless of whether the calculated SUVmax shows heterogeneous changes (11,20).
The aim of this study was to evaluate the calculation of VOI, with the software tools of the PET/CT Siemens Biograph 16, in the measurement of volume radioactivity and to differentiate necrotic sites from residual tumor activity in a phantom. The results will help to design a method to evaluate therapeutic procedures for solid tumors that will be implemented at the Instituto Nacional de Cancerología in Mexico City (INCan).
MATERIALS AND METHODS
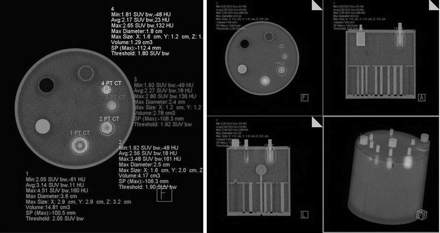
To perform this study, we used an Esser Flangeless PET Phantom (Technology Imaging Services), which meets the requirements set by the American College of Radiology. The main applications of this phantom include the evaluation of tumor detectability, SUVs, and volume sensitivity. The phantom consists of an acrylic cylinder with volume of 6.4 L (5.7 L with inserts). The interior of the cylinder can be filled with 3 sections of different inserts that represent objects with (hot) or without (cold) activity concentrations. The first section consists of 4 refillable thin-walled cylinders that are filled with radioactive material to represent hot volumetric regions. The second section consists of 6 solid spheres of different diameters that represent cold volumetric regions. Finally, the third section consists of an array of solid rods that represent a mixed distribution of cold and hot regions (Fig. 1).
Esser Flangeless PET Phantom with inserts.
The phantom with all the inserts was filled with 5.7 L of distilled water having an activity of either 210.9 or 632.7 MBq (5.7 or 17.1 mCi) of 18F-FDG to get a background concentration of 37 or 111 kBq (1 or 3 μCi)/mL, respectively. The 4 refillable cylinders were also filled with distilled water and 18F-FDG to get 222 or 666 kBq (6 or 18 μCi)/mL in each, to maintain the same proportion (1:6) between background and cylinder activities. The VOI was quantified in 2 different sessions, conducted on different days by the same investigator (a nuclear physician dedicated to PET/CT diagnosis) after PET/CT had been performed on the phantom with a Siemens Biograph 16 (Fig. 2; a color version of this figure is available as a supplemental file online at http://tech.snmjournals.org/). The goal of this procedure was to evaluate the VOI measurements as a function of the activity concentration in the phantom. TrueD software by Siemens was used to build the VOI and determine the volume radioactivity of the cylindric inserts (cm3) and to compare it with the real volume reported by the manufacturer. Briefly, the observer previously corroborated the locations of regions of activity concentration (the inserts) in the sagittal, coronal, and axial planes of the PET image, and the VOI tool of the TrueD software was used to automatically draw a margin around the inserts in each plane. Then, in the fusion image (PET/CT), the threshold tool was used to adjust the VOI to the edges of the inserts and cover the totality of the lesion observed in the CT image. The VOI values reported by TrueD were used in the analysis.
VOI quantification in cylindric inserts of phantom filled with 222 kBq (6 μCi) of 18F-FDG per milliliter. Background activity was set at 37 kBq (1 μCi)/mL. TrueD software was used to build VOI and determine volume radioactivity of cylindric inserts.
The VOI technique was also used to discern mixed distributions of cold and hot regions of 18F-FDG activity, which represent areas of necrosis without tumor activity. The VOI was measured in the third section of the phantom to discern volumes without activity (e.g., number of cold rods of different diameters located inside the VOI) (Fig. 3; a color version is available as a supplemental file online). The VOI was built as described before, and VOI measurements were performed on each group of rods of different diameters. Threshold was adjusted in each measurement to include only rods of the same size. The percentage of cold rods discerned with the VOI technique was obtained by dividing the number of cold rods inside the VOI by the actual number of rods and multiplying by 100.
VOI measured in third section of phantom to discern volumes without activity (e.g., number of cold rods of different diameters inside VOI).
To evaluate the probability of observing a difference in means, the cylinder volumes measured in the first and second sessions were averaged and compared with the actual reported volumes by t test analysis. The relationship between the percentage discerned in the first and second sessions and the size of the cold rods was evaluated with Spearman nonparametric correlation. The confidence interval was 95%.
RESULTS
The average VOI of the cylinders measured in the first and second sessions, as well as the actual volume in each cylinder, is shown in Table 1. Volumes measured with the VOI technique were similar to the actual volumes of cylinders in the phantom (no statistical difference, P > 0.05 after t test analysis). The percentage of cold rods discerned with the VOI technique, and the ability of the technique to identify rods without 18F-FDG activity in the first and second sessions, is shown in Table 2. The increment in background activity (from 1 to 3 μCi/mL) made it easier to discern the 4.8-mm rods in the second session; however, the lower percentage of 7.9-mm rods identified during the second session was associated with normal variability in the measurements. The Spearman correlation between the size of the rods without 18F-FDG activity with the percentage discerned in the first and second sessions was 0.984 (P < 0.01) (Table 2).
Comparison of Actual Cylinder Volumes and Cylinder VOIs
Correlation Between Size of Rods Without 18F-FDG Activity and Percentage Discerned by VOI Technique
DISCUSSION
The results have shown that VOI technique is capable of measuring the volume radioactivity of the different cylinders in the phantom with good accuracy. The proportion of activity in the first measurement to that in the second measurement was 1:3, and the proportion of activity in the background to that in the cylinders was kept to 1:6. The VOI measurements were seen to be independent of the activity concentration in the inserts and in the background and were close to those reported by the manufacturer. The potential benefit of this tool is the ability to get an objective value for tumor volume in cubic centimeters, which may allow comparison of the final volume of the basal tumor. In addition, lesions that were previously nonmeasurable because their margins could not be defined can be evaluated by determining the extent of their infiltration (21). Other authors have reported the use of the VOI technique on phantoms. Boucek et al. (17) have applied a phantom with hot spheres to compare VOI with SUVmax using different techniques, including the adaptive threshold as was done in our study, with results similar to ours. Also, Green et al. reported a novel method to calculate VOI in a phantom—a method that achieved adequate reproducibility (22).
The correlation between the highest caliber of rods without 18F-FDG activity and the highest rate of detection by the VOI technique was good; in both sessions, 100% of the cold rods 12.7, 11.1, and 9.5 mm in size could be visualized. Because at least 76% of the 7.9-mm rods could be visualized, we propose that detection of this size be referred to as positive visualization by VOI technique. The VOI technique is a complement to SUVmax that can estimate the efficiency of cancer therapy through an approximation of the integral of glycolytic activity of 18F-FDG in a tumoral volume (18,19,22). In patients with several lesions, the VOI technique can be added to clarify the total tumoral volume. Also the VOI technique can determine the tumoral activity in cubic centimeters to assess a heterogeneous response to treatment (necrotic areas associated with residual tumor activity) and can be useful in lesions with circumferential or infiltrative growth without precise limits (11).
The VOI technique can assess the total tumor activity of a malignancy that includes areas of necrosis and areas of persistent disease that have responded to treatment. There are reports of the use of VOI technique to evaluate patients with various tumors, such as mesotheliomas, colon cancer, and lung cancer, among others (17,23,24). Besides being used for a small number of VOI evaluations on the phantom, these measurements took into account all the factors that could affect accuracy and reliability. The next step is to conduct further studies involving cancer patients, to gain deeper knowledge of this tool in clinical practice and compare the VOI technique with SUVmax in the evaluation of the total tumor glycolytic activity of a neoplasm in response to treatment.
CONCLUSION
With a phantom, the VOI technique was able to determine volumes of 18F-FDG activity—mimicking sites of tumor activity—as small as 1.9 cm3. In areas without radioactivity—mimicking areas of necrosis—the VOI technique identified 100% of 9.5-mm rods and 76% of 7.9-mm rods. The VOI technique has shown potential in evaluating the tumor activity of a malignancy that includes areas of necrosis and in evaluating treatment response in regions with persistent disease. Clinical studies should be performed to evaluate this potential.
Acknowledgments
We gratefully acknowledge Ulises Berry, NMT, and Flavio Trujillo for their assistance with this study.
REFERENCES
- Received for publication April 22, 2010.
- Accepted for publication October 29, 2010.