Abstract
In the operation of any SPECT/CT system, in addition to internal radiation exposure (γ-ray) resulting from administration of radiopharmaceuticals, external radiation exposure (x-ray) from the CT device has to be taken into consideration in the light of recommendations from the International Commission on Radiological Protection. These recommendations include justification of practices (the use of radiation produces sufficient benefit to offset any risks caused by the use of radiation), optimization (the incurred exposure by the use of radiation should be kept as low as reasonably achievable), and dose limitation. The internal radiation exposures of each organ after administration of radiopharmaceuticals are calculated by the MIRD Committee method. For example, the internal radiation exposure index for brain perfusion scintigraphy is 0.8 mGy/37 MBq for N-isopropyl-4-iodoamphetamine(123I) hydrochloride or 0.19 mGy/37 MBq for ethyl cysteinate dimer. On the other hand, the external radiation exposure from a CT device is calculated using the CT dose index volume (CTDIvol)—a measured and calculated value unique to the CT scanner and scan parameters used—and a dose–length product, which is obtained from CT conditions and generally used as a reference value for CT radiation exposure. However, CTDIvol and dose–length product are calculated values unique to each device, not the value of external radiation exposures of each organ. Therefore, we believe that it is necessary to measure the total (internal plus external) radiation exposure dose from CT. In the present study, using an anthropomorphic phantom for deep-body total absorbed dose measurement, we evaluated the radiation exposure doses (organ-absorbed doses) of each organ under various CT conditions. Methods: The radiation exposure doses of each organ were measured by inserting thermoluminescent dosimeter elements into the phantom under various CT conditions. Results: The following were brain radiation exposure doses in the head region. For 90 kVp and 25 mAs, 1.39 mGy (CTDIvol, 1.8 mGy), for 90 kVp and 300 mAs, 17.00 mGy (CTDIvol, 21.2 mGy), for 120 kVp and 25 mAs, 3.21 mGy (CTDIvol, 3.8 mGy), for 120 kVp and 300 mAs, 37.79 mGy (CTDIvol, 47.7 mGy), for 140 kVp and 25 mAs, 5.08 mGy (CTDIvol, 5.5 mGy), and for 140 kVp and 300 mAs, 65.07 mGy (CTDIvol, 65.6 mGy). The eye radiation exposure doses were as follows. For 90 kVp and 25 mAs, 1.94 mGy (CTDIvol, 1.8 mGy), for 90 kVp and 300 mAs, 20.31 mGy (CTDIvol, 21.2 mGy), for 120 kVp and 25 mAs, 3.71 mGy (CTDIvol, 3.8 mGy), for 120 kVp and 300 mAs, 49.72 mGy (CTDIvol, 47.7 mGy), for 140 kVp and 25 mAs, 5.44 mGy (CTDIvol, 5.5 mGy), and for 140 kVp and 300 mAs, 69.76 mGy (CTDIvol, 65.6 mGy). In addition, the radiation exposure doses of the cervical, thoracic, abdominal, and pelvic regions were measured in detail. Conclusion: Our estimated external radiation exposure doses (x-ray) of each organ under various CT conditions, along with the internal radiation exposure doses (γ-ray) resulting from the administration of radiopharmaceuticals, seem to be useful as reference values in understanding the radiation exposure doses for performing various nuclear medicine examinations.
- SPECT/CT
- MIRD method
- exposure dose
- thermoluminescent dosimeter (TLD)
- Alderson RANDO anthropomorphic phantom
Recently, with the introduction of hybrid imaging devices such as PET/CT and SPECT/CT modalities, many reports describing the principles of the CT device (1,2), and some clinical studies (3–12), have been published. Furthermore, there are also reports on the radiation exposure to radiologists (13–17) in the operation of such devices and the measurement techniques of radiation exposure (18). However, none of these reports addressed in detail the radiation exposure to the patients.
In the operation of hybrid imaging modalities in the field of nuclear medicine, in addition to internal radiation exposure (γ-ray) resulting from the administration of radiopharmaceuticals, external radiation exposure (x-ray) from a CT device has to be taken into consideration in the light of recommendations from the International Commission on Radiological Protection (ICRP) (19–20). These recommendations include justification of practices (the use of radiation produces sufficient benefit to offset any risks caused by the use of radiation), optimization (the incurred exposure by the use of radiation should be kept as low as reasonably achievable), and dose limitation. The internal radiation exposure doses of each organ after radiopharmaceutical administration are calculated by the MIRD method (21). On the other hand, for the external radiation exposure dose from a CT device, CT dose index volume (CTDIvol), which is calculated from the CT conditions, is used as a reference value (18,22) for CT radiation exposure. This is a calculated value unique to each device, not the value of external radiation exposures of each organ. Therefore, we believe that it is necessary to measure the detailed radiation exposure from CT. In the present study, using an Alderson RANDO anthropomorphic phantom (RAN-100; Phantom Laboratories), we estimated absorbed doses of x-rays of each organ under various CT conditions.
MATERIALS AND METHODS
Measurement of CTDIvol Using CT Phantom
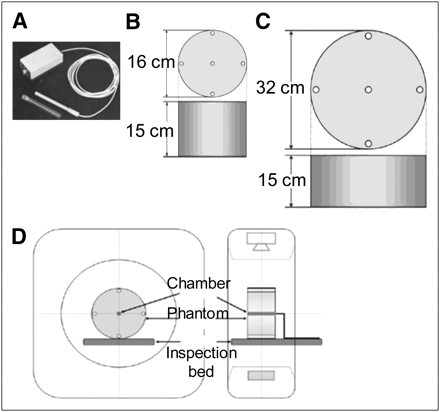
A 16-slice CT device loaded in a Precedence SPECT/CT system (Philips) was used. In addition, the following devices were used: a radiation monitor (model 9015, 10x5-3CT; Radcal) (Fig. 1A) for measurements, polymethylmethacrylate (PMMA) cylindric head phantom (15 × 16 cm; Fig. 1B) as a pencil-shaped ionization chamber dosimeter, PMMA cylindric abdominal phantom (15 × 32 cm; Fig. 1C), and Baromex (Sato Keiryoki MFG) system as a hygrometer/thermometer. The ionization chamber dosimeter and hygrometer/thermometer were calibrated according to the method of the Japan Quality Assurance Organization.
(A) Pencil-shaped ionization chamber dosimeter. (B) PMMA cylindric head phantom. (C) PMMA cylindric abdominal phantom. (D) Layout of measurement system setup.
Figure 1D shows the layout of the measurement system setup. For the CT conditions, tube voltages of 90, 120, and 140 kVp were used, and tube current-times of 25, 50, 100, 150, 200, 250, and 300 mAs were applied at each tube voltage to obtain a CTDIvol for each imaging condition. We measured the CTDIvol of a CT device in accordance with the method of Goldman (18). The CTDIvols (index values) obtained from the scan device were compared and analyzed with the actual measured values.
Absorbed Doses of Each Organ Using Anthropomorphic Phantom
The Precedence SPECT/CT system (Philips) was used. In addition, we used a TLD reader (UD-512P; Panasonic) and TLD heat-treatment furnace (UD-606P; Panasonic) as measurement devices, a model UD-170L TLD (Panasonic), and a Baromex hygrometer/thermometer. The pencil-shaped ionization chamber dosimeter and hygrometer/thermometer was calibrated according to the method of the Japan Quality Assurance Organization.
The same tube voltages and currents as were used for CTDIvol were used to obtain radiation exposure doses by inserting 30 units of TLDs each at the head and cervical, thoracic, abdominal, and pelvic regions of the phantom (Fig. 2) under each imaging condition.
Alderson RANDO anthropomorphic phantom for deep-body total absorbed dose measurement.
TLD Protocol
The specifications for the TLDs were as follows: model, UD-170L; luminescent material, beryllium oxide element; size, 1.2 mm; diameter, 8 mm; measuring range, 200 μSv to 20 Sv; and measuring radiation type, x-ray and γ-ray. After exposure to 400°C for 1 h and at 100°C for 6 h in an annealing oven (TLD heat-treatment furnace), TLDs were used for the CT scan and the data were collected using a TLD reader.
An organ-absorbed dose was calculated by multiplying the mean of the TLD readouts by a correction coefficient and a tissue dose–conversion coefficient (23) for each organ against air at the effective energy of the tube voltage applied. The radiation exposure dose (organ-absorbed dose) was derived from the absorbed dose of each organ (19). In addition, before the study, correction coefficients were obtained by comparing the measured values for each effective energy calculated with a CT device using an ionization chamber dosimeter (model 9015; Radcal) with the TLD measured values.
RESULTS
Comparison of Measured Values and Index Values of CTDIvols
Figures 3A and 3B show the actual measured and index values. In the PMMA cylindric head phantom, there was not much difference at 90 kVp. At 120 and 140 kVp, the index values were lower than the actual measured values as the current increased. In the PMMA cylindric abdominal phantom, the index values were lower than the actual measured values as the current increased at 90 and 120 kVp, but there was not much difference at 140 kVp.
CTDIvol measurement results of PMMA cylindric head phantom (A) and cylindric abdominal phantom (B) at each tube voltage and current. Actual V = actual measured values; Index V = index values.
Absorbed Doses of Each Organ Using Anthropomorphic Phantom
Figure 4 shows the measured and index values of the brain, eyes, thyroid glands, and lungs. Scans of the brain and eyes in the head region were acquired under the same conditions. The measured values of the brain gradually deviated from the corresponding index values as the current increased, whereas no deviations from the index values were observed for the measured values of the eyes as the current increased. Although the scans were obtained under the same condition, the values did not show a similar trend. In the cervical region (thyroid glands), the scans were acquired in such a way as to include the thoracic region. Accordingly, the CTDIvols of the cervical region were similar to those of the thoracic region.
TLD measurement results of each organ in anthropomorphic phantom: head region, brain (A); head region, eyes (B); cervical region, thyroid glands (C); thoracic region, lungs (D). Bra = Brain; Thy = Thyroid glands.
Figure 5 shows the measured and index values of the mediastinum, skin of the thoracic region, liver, and kidneys. Although the scans were acquired under the same conditions on the skin surfaces of the thoracic (Fig. 5B) and pelvic (Fig. 6C) regions, there were differences in the TLD values between them.
TLD measurement results of each organ in anthropomorphic phantom: thoracic region, mediastinum (A); thoracic region, skin (B); abdominal region, liver (C); abdominal region, kidneys (D). Medi = mediastinum; Kid = kidneys; Live = Liver; Sk1 = skin of thoracic region.
TLD measurement results of each organ in anthropomorphic phantom: pelvic region, urinary bladder (A); pelvic region, muscles (B); pelvic region, skin (C). Blad = urinary bladder; mus = muscles; Sk2 = skin of pelvic region.
Figure 6 shows the measured and index values of the urinary bladder, muscles, and skin of the pelvic region.
Figure 7 shows the measured and index values of the small intestine, large intestine, bones (red bone marrow), and ovaries.
TLD measurement results of each organ in anthropomorphic phantom: abdominal region, small intestine (A); abdominal region, large intestine (B); abdominal region, bones (C); pelvic region, ovaries (D). Bon = bones; La_int = large intestine; Sm_int = small intestine; Ova = ovaries.
Tables 1–3⇓⇓ show calculated TLD value–to–CTDIvol ratios. In the head region, the TLD values of were lower than the CTDIvols (TLD value–to–CTDIvol ratio, 0.93 ± 0.12), whereas in the cervical, thoracic, abdominal, and pelvic regions, the TLD values were higher than the CTDIvols (TLD value–to–CTDIvol ratio, 1.78 ± 0.41) under each CT condition.
Calculated TLD-to-CTDIvol Ratios for Head Region
Calculated TLD-to-CTDIvol Ratios for Cervical and Thoracic Regions
Calculated TLD-to-CTDIvol Ratios for Abdominal and Pelvic Regions
Table 4 shows MIRD and TLD values of total (internal plus external) radiation exposure in the brain and liver, Appendices 1–5⇓⇓⇓⇓ show the radiation exposure doses by the MIRD method; Tables 1–3⇑⇑ also show radiation exposure doses measured using TLDs. These results suggest that detailed radiation exposure doses can be obtained by adding the MIRD and TLD values. In addition, we could obtain the detailed radiation exposure doses in a similar manner for other nuclear medicine modalities as well.
MIRD and TLD Values of Total Radiation Exposure in Brain and Liver
Exposure Doses in Head Region According to MIRD Method
Exposure Doses in Cervical Region According to MIRD Method
Exposure Doses in Thoracic Region According to MIRD Method
Exposure Doses in Abdominal Region According to MIRD Method
Exposure Doses in Total Body According to MIRD Method
DISCUSSION
In the present study, we evaluated the radiation exposure doses from a CT device loaded in the hybrid imaging system. Although the imaging conditions in the study of Goldman (18) were 120 kVp and 300 mAs (number of settings, 1) for the PMMA cylindric head phantom and 120 kVp and 250 mAs (number of settings, 1) for the PMMA cylindric abdominal phantom, we used 21 different imaging condition settings with the PMMA cylindric head phantom and 21 different imaging condition settings with the PMMA cylindric abdominal phantom in the present study. Consequently, the index values of the CTDIvols were lower than the actual measured values under each imaging condition. It is likely that CTDIvol was underestimated because x-rays with a wider beam width were used in multidetector CT and the formulas programmed in the system vary. Furthermore, the measurement method of radiation exposure doses described by Goldman (18) was for the device, not for human bodies. In the light of our present study, radiation exposure doses should be used as a reference only after the actual values of CTDIvol are measured and the specific features of each device are understood.
Guillet et al. (16) measured the radiation exposure of fingers from a PET device, Deloar et al. (17) measured the body surface radiation exposure using a MIRD phantom and TLDs from a PET device, and Lundberg et al. (13) measured the daily radiation exposure on the skin surface from a SPECT device (high-exposure modalities: gated heart-pool [900 MBq] and methoxyisobutylisonitrile stress [1,000 MBq] scans, 1.5–2 μSv/h; low-exposure modalities: 201Tl rest cardiac [40 MBq] and thyroid [150 MBq] scans, 0.2–0.4 μSv/h). However, these studies concerned occupational radiation exposures to the radiologists after administration of radiopharmaceuticals not radiation exposures to patients. Furthermore, there is currently no report on the radiation exposure from the CT component of the hybrid imaging system.
In the field of nuclear medicine, the internal radiation exposure doses of each organ after radiopharmaceutical administration are calculated by the MIRD method. For example, an internal radiation exposure index for brain perfusion scintigraphy is 0.8 mGy/37 MBq for N-isopropyl-4-iodoamphetamine(123I) hydrochloride or 0.19 mGy/37 MBq for ethyl cysteinate dimer (Appendices 1–5⇑⇑⇑⇑). On the other hand, regarding the external radiation exposure from a CT device, CTDIvol, dose–length product (24), and multiple-scan average dose—which is obtained from the CT condition—are generally used as reference values for CT radiation exposure. These are calculated values unique to each device and cannot be considered as an external radiation exposure of each organ. However, CTDIvol can be used as a reference of radiation exposure dose of the subject and is quite useful in managing the radiation exposure level. Actually, the International Atomic Energy Agency (IAEA) and ICRP adopt CTDI or multiple-scan average dose values as reference values of radiation exposure for the head CT and use them in their reports (IAEA, 50 mGy [multiple-scan average dose]; ICRP, 60 mGy [CTDI100; the International Electrotechnical Commission definition of CT dose]; and Japan Association of Radiological Technologists, 65 mGy [CTDIvol]). According to the results of our present study, the imaging conditions matching the radiation exposure reported by the IAEA, ICRP, and Japan Association of Radiological Technologists are higher than 300 mAs at 120 kVp and correspond to 250–300 mAs at 140 kVp. The imaging condition of the head (in adults) in the actual clinical settings is 120 kVp (tube voltage) and 200–300 mAs, and under this imaging condition, the radiation exposure dose and CTDIvol are 28.81–49.72 mGy and 30.4–47.7 mGy, respectively. It has been recognized that the reference values of each tissue tend to be overestimated, as compared with their corresponding radiation exposure doses (Tables 1–3⇑⇑). In addition, CTDIvols were underestimated, except for those in the brain region. Our results clarified that the radiation exposure dose of TLDs calculated using a phantom for deep-body total dose measurement should be used as a reference value of radiation exposure in the patients (Tables 1–3⇑⇑).
ICRP (25) recommends maintaining the appropriate balance between the medical benefits and risks of potentially inducing cancer in patients at a dose level produced by a CT examination. Considering this recommendation, CTDIvol is not sufficient to understand the absorbed dose of a specific organ, and it is thus difficult to take appropriate precautions against the risks of inducing cancer. Therefore, it is necessary to assess the absorbed dose at the organ level. In the present study, we assessed the absorbed doses at an organ level by changing the tube voltages and currents. To obtain radiation dose estimates without restriction to specific CT devices from various manufacturers, we did not use the CT exposure-reducing technology (DoseRight automatic current selection, DoseRight dose modulation, DoseRight electrocardiogram-gated modulation, and such [all from Phillips]; Appendix 6) loaded in the CT device in our present study. With this employment, our present results can be used as references for the radiation exposure doses of each organ without restriction to a specific CT device manufacturer and can be applied to any CT device loaded in a PET/CT system as well.
Philips Dose-Management List
CONCLUSION
Estimation of the external radiation exposures (x-ray) of each organ under a certain CT condition, along with internal radiation exposure (γ-ray) resulting from the administration of radiopharmaceuticals, helps us understand the detailed radiation exposure from various nuclear medicine modalities (SPECT/CT) and seems to be useful as a reference value of radiation exposure for performing these examinations. In addition, this information is also useful for explaining the examinations to the patients.
Acknowledgments
We thank Teruhiko Takayama, Kayo Hara, Kohana Hara, Konomi Hara, and all the radiologic technologists of Sumitomo Hospital for technical support.
REFERENCES
- Received for publication February 3, 2010.
- Accepted for publication May 14, 2010.