Abstract
Objective: Our study evaluated the accuracy and reliability of 3 radiochemical purity (RCP) measurement methods of 99mTc-sestamibi. A regular-sized (1.0 cm × 9.0 cm) Whatman 31 ET Chr paper strip (regular 31 ET) also was included in our evaluation because of its ease in handling. Methods: The miniaturized and regular 31 ET methods were compared with the standard RCP testing method (aluminum oxide–coated plastic thin-layer chromatography [TLC] plate, with ≥ 95% ethanol as the developing solvent). A total of 30 experimental runs were performed in triplicate (n = 90) over an RCP range of 82%–98%. The 99mTc-sestamibi preparations were reconstituted purposely to ensure that 50% of the tested samples had RCP values below the 90% limit. Results: The evaluated RCP ranges were 89.9% ± 6.3%, 91.0% ± 3.8%, and 91.4% ± 4.3% for the TLC, miniature 31 ET, and regular 31 ET methods, respectively (n = 30 each). A strong correlation was found between the TLC and miniature 31 ET methods (r = 0.92), as well as between the TLC and regular 31 ET methods (r = 0.94). Both alternative methods tended to overestimate RCP value as determined by the TLC method, especially in an RCP range below 95%. This resulted in a false-positive rate of 27% for the miniature 31 ET method and 33% for the regular 31 ET method. The test/retest reliability was 99% for both the TLC and regular 31 ET methods, and 91% for the TLC and miniature 31 ET methods. Conclusion: The miniature and regular 31 ET methods produced a high false-positive rate, which makes them unacceptable for the determination of RCP value of 99mTc-sestamibi.
- radiochemical purity testing
- quality control
- technetium-99m-sestamibi
- thin-layer paper chromatography
- nuclear pharmacy
The package insert of Cardiolite (DuPont Pharmaceuticals Co., Billerica, MA) recommends using a thin-layer chromatography system with an aluminum oxide–coated, plastic thin-layer chromatography (TLC) plate as the stationary phase and ≧95% ethanol as the mobile phase for determining the radiochemical purity (RCP) of 99mTc-sestamibi (1). This RCP testing method is a lengthy and involved process that can take up to 30–40 min to complete (2). This is an unacceptable length of time for determining RCP in a busy laboratory or in emergency situations (2).
Several alternative RCP testing methods for 99mTc-sestamibi have been proposed (2–10). These systems are generally faster and more practical for the clinical setting. Our laboratory currently uses an alternative method that was proposed by Hung et al. in 1991 (2). It is a miniaturized paper chromatography system that uses Gelman Solvent Saturation Pads (Gelman Sciences, Ann Arbor, MI) as the stationary phase and chloroform:tetrahydrofuran (THF) (1:1, v/v) mixture as the mobile phase. This method is quick (3–4 min) and effective (2). However, there are two disadvantages of this RCP testing system.
First, there are some concerns about the safety of the solvents used in the process. People tend to have reservations about handling chloroform and THF. The second concern of this RCP testing system is the mobile phase. This is a mixture of two chemical solvents that has to be prepared biweekly and refrigerated because of the volatile nature of both chemicals and the necessity to maintain 1:1, v/v ratio. This 1:1 ratio is critical for an adequate separation of 99mTc-sestamibi from the radiochemical impurities. If a preparation of 99mTc-sestamibi fails this alternative RCP testing, it is not completely clear if it is the kit or the solvent that is at fault. This special solvent preparation and maintenance (i.e., weekly preparation and refrigerated storage) is a somewhat cumbersome process.
In 1991 Zimmer et al. (6) proposed a method for RCP testing of 99mTc-sestamibi that uses Whatman 31 ET Chr chromatography paper (Whatman Inc., Clifton, NJ) as the stationary phase and ethyl acetate as the mobile phase. A 1-solvent RCP method would be a great improvement over the solvent mixture system (e.g., chloroform:THF). Unfortunately, no results or experimental methods were included in the original article for the evaluation and support of the proposed method. The purpose of this study was to evaluate the validity of this alternative RCP testing method.
MATERIALS AND METHODS
Testing of RCP Levels of Technetium-99m-Sestamibi
To accurately determine the usefulness of an alternative method, it must be evaluated against the standard TLC system over a wide range of RCP values as well as replication of trials (11). This experiment consisted of a total of 30 comparison trials, and each trial was performed in triplicate measurements (n = 90). To ensure the pass/fail range would be carefully evaluated, the RCP of 99mTc-sestamibi samples was carefully controlled. We evaluated RCP values between 82% and 98%; half of the samples were above and half of the samples were below the critical 90% value. This was arranged by combining a small aliquot of 99mTc-sestamibi with varying amounts of 99mTc-sodium pertechnetate. The solution was properly mixed and the desired RCP preparations were available for evaluation. A pipette was used to consistently apply small drops (20 μL) of 99mTc-sestamibi on the chromatographic media (i.e., Whatman 31 ET Chr paper and TLC plate). All RCP testing was performed in triplicate to obtain estimates of the reliability of each method.
Standard TLC Method
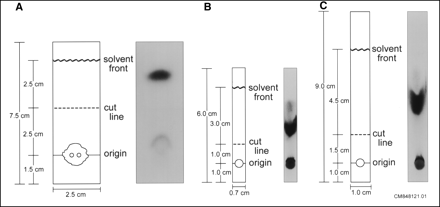
The recommended RCP testing method, as described in the Cardiolite package insert (1), was used as a standard method (Fig. 1A).
Chromatography diagrams and autoradiographs of (A) TLC, (B) mini 31 ET, and (C) regular 31 ET methods for RCP analysis of 99mTc-sestamibi.
Miniaturized Whatman 31 ET Chr Paper with Ethyl Acetate (Mini 31 ET)
This system involves the use of a miniaturized Whatman 31 ET Chr strip (0.7 cm × 6.0 cm) as the stationary phase and ethyl acetate as the mobile phase (Fig. 1B) (6). A 20-μL drop of 99mTc-sestamibi was placed at the origin, 1 cm from the bottom of the strip (Fig. 1B). The strip was then placed in an ethyl acetate developing chamber and left undisturbed for 1.0–1.5 min, until the solvent front reached the appropriate endpoint (i.e., 5 cm from the bottom of the strip) (Fig. 1B). The bound 99mTc-sestamibi migrated to the top of the strip, while any 99mTc impurities remained at the bottom. The procedure required the strip to be cut 2 cm up from the bottom immediately after chromatography separation (Fig. 1B).
Regular Whatman 31 ET Chr Paper with Ethyl Acetate (Regular 31 ET)
The method is the same preparation as the published mini 31 ET method, except that the strip size was increased to 1.0 cm × 9.0 cm with the origin, cut line, and solvent front at 1.0 cm, 2.5 cm, and 7.0 cm from the bottom of the strip, respectively (Fig. 1C). This larger size strip was evaluated because of its ease in handling. Moreover, it was hypothesized that the larger area would allow for a greater separation and, therefore, more accurate results (Fig. 1C).
Statistical Analyses
The test/retest reliability of each of the RCP testing methods was estimated as the ratio of between-trial variance to the total variance observed (i.e., between- + within-trial variances). The correlation and calibration of the 2 proposed methods relative to the standard TLC method was investigated with the linear regression framework using the mean of the 3 replicated TLC estimates as the gold standard. The false-positive rate of the new methods were based on the number of trials in which the new method had a mean value above 90% when the TLC method had a mean value below 90%.
RESULTS
Mini 31 ET Versus TLC
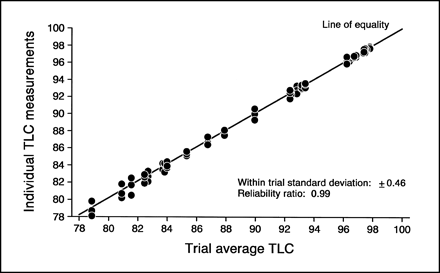
The developing times for the mini 31 ET strips were 1.0–1.5 min, much faster than the 10–15 min developing times of the TLC method (1,2). The test/retest reliability of the mini 31 ET was 91% (Fig. 2). There was one trial that was more varied than the others (Fig. 2). When this was accounted for the percentage was increased to 99% (Fig. 2), the same as the TLC retest/reliability (Fig. 3). Over the RCP range tested, there was a strong correlation between the mini 31 ET and the TLC as determined by the Pearson correlation (r = 0.92) (Fig. 4). However, when this was corrected for the mini 31 ET's deviation from the line of equality produced by the TLC, the correlation was reduced to a less significant value (r = 0.80) (Fig. 4). The mini 31 ET also tended to produce falsely high RCP results. This overestimation resulted in a 27% false-positive rate.
Retest/reliability of the mini 31 ET RCP testing method (30 trials with triplicate samples in each trial). Numbers in parentheses (i.e., ±0.47 and 0.99) were derived from excluding trials with wide variation (29 trials with triplicate samples in each trial).
Retest/reliability of the TLC RCP testing method (30 trials with triplicate samples in each trial).
Linear regression analysis between the mini 31 ET method and the standard TLC method (30 trials with triplicate samples in each trial).
Regular 31 ET Versus TLC
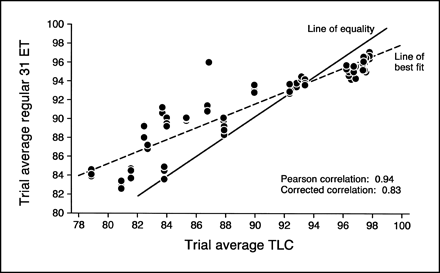
The developing times for the regular 31 ET were between 3–4 min, slightly longer than the mini 31 ET strips, but a marked improvement over the TLC method (1,2). These strips, like the mini 31 ET, also produced the same retest/reliability as the TLC, 99% (Figs. 3 and 5). A strong correlation between the regular 31 ET and TLC was determined by the Pearson correlation (r = 0.94) (Fig. 6). However, when the deviation from the TLC's line of equality was taken into consideration, the correlation was reduced (r = 0.83) (Fig. 6). Although this method has a somewhat stronger correlation with the TLC than the mini 31 ET, it produced an even higher false-positive rate, 33%. Figure 6 displays the overshoot and its inability to correlate in a useful manner.
Retest/reliability of the regular 31ET RCP testing method (30 trials with triplicate samples in each trial).
Linear regression analysis between the regular 31 ET method and the standard TLC method (30 trials with triplicate samples in each trial).
DISCUSSION
Although the Gelman Solvent Saturation Pads/chloroform:THF (1:1, v/v) system is quite fast and effective in the determination of a RCP value of 99mTc-sestamibi (2), people are skeptical about the handling of chloroform and THF. A booklet, “Alternative Radiochemical Purity Testing Procedures for the Compounded Radiopharmaceuticals Approved from 1988–1997,” was published by the American Pharmaceutical Association in 1998 and evaluated the safety of some common chemical solvents based on a 10-point scale (11). These safety ratings were based on both the National Fire Protection Association and Baker SAF-T-DATA chemical solvent safety rating systems (12,13). These systems take into account four hazard categories: biologic effects, flammability, chemical reactivity, and impact of physical contact that may result from handling the materials. The scores awarded each solvent by these systems were converted to a 10-point scale, 10 being the safest (water = 10) and 0 being the most hazardous. Chloroform and THF received scores of 6.3 and 5.0, respectively (3). This demonstrates that the solvents pose no real health risk; however, there is still the issue of people's perceptions of their safety.
The Whatman 31 ET Chr paper and ethyl acetate system has many advantages over the recommended TLC method (1) as well as the Gelman Solvent Saturation Pads/chloroform:THF method (2). One of the main advantages of this alternative RCP testing system (i.e., Whatman 31 ET Chr paper as a stationary phase and ethyl acetate as a mobile phase) is that the time required to complete the testing is relatively short. Developing time for the mini 31 ET can be up to 15 times faster than the standard TLC method (1,2) and up to four times faster than the RCP testing system of the Gelman Solvent Saturation Pads/chloroform:THF method (2). This time saving is even more dramatic if one takes into account the time invested in predrying the TLC plate (1 h) (1) and drying the sample spot (∼ 15 min) (1,2).
Another advantage of this proposed system is the safety and convenience of the solvent. On the solvent safety scale, from the “Alternative Radiochemical Purity Testing Procedures for the Compounded Radiopharmaceuticals Approved from 1988–1997,” ethyl acetate received a score of 5.0, which is safer than ethanol (safety score = 3.1) used in the TLC method (11). Ethyl acetate is somewhat less safe than the chloroform and THF (average safety score = 5.65) (3). However, because ethyl acetate is a single solvent that requires no special preparation or storage, it would be an improvement over our current system.
There is one major drawback to this proposed system of RCP testing: the high false-positive rate. Up to 27% of 99mTc-sestamibi samples tested with the mini 31 ET method and up to 33% of sample preparations tested with the regular 31 ET method could actually be below the 90% RCP limit. If a failed 99mTc-sestamibi preparation was accepted for clinical use (because of the false-positive RCP result), this could affect the imaging quality, as well as cause an unnecessary radiation dose to other nontarget organs of the patient. A lower RCP could result in a lower count study and decreased image quality. It also could alter the organ dosimetry of 99mTc-sestamibi, which may pose unnecessary radiation exposure risks to the patient.
CONCLUSION
With a single paper strip and one chemical solvent, the Whatman 31 ET Chr paper chromatography/ethyl acetate system would be a quicker and less cumbersome RCP testing method for 99mTc-sestamibi. Unfortunately, it is unacceptable because of the high false-positive rate associated with both the mini 31 ET or regular 31 ET method.
ACKNOWLEDGMENTS
The authors thank Vicki S. Krage and Rose M. Busta for their assistance in the preparation and submission of this paper. This paper was presented at the 46th Annual Meeting of the Society of Nuclear Medicine, Los Angeles, CA, on June 8, 1999.
Footnotes
For correspondence or reprints contact: Joseph C. Hung, PhD, BCNP, Nuclear Medicine, Department of Radiology, Mayo Clinic, 200 First St. SW, Rochester, MN 55905; Phone: 507-284-4399; E-mail: jhung{at}mayo.edu.